| Open Access | Peer Reviewed | Original Research |
Evaluation of Economically Important Cultivars of Seed Potato for Minituber Production
Fazal Rehman*, Muhammad Shah Zaman, Muhammad Khalid, Sohailur Rehman and Abdul Noor
ABSTRACT
Experiments were conducted to evaluate mass production of potato minitubers by using in vitro produced plants and sprout cuttings under Gilgit Baltistan conditions. The experiments were arranged in Complete Randomized Design and replicated three times. Four potato cultivars ‘Roko’, ‘Bartina’, ‘Barna’ and ‘Kuroda’ multiplied in vitro through nodal cuttings were planted in a greenhouse. The cultivars showed variation in different parameters studied. Results revealed that cv. ‘Kuroda’ showed greater plant height (103.7 cm), higher total tuber weight per plant (194 g) and the maximum number of minitubers per plant (21.9) as well as total number of minitubers per plot (14,235) from 650 in vitro produced plants, whereas shorter plant height (33.4 cm), lower tuber weight per plant (102.8 g) and the minimum number of minitubers per plant (9.4) and total number of minitubers per plot (6,110) were observed in cv. ‘Bartina’. However, the cultivar Bartina’ produced the maximum number of stems per plant (2.2), while cv. ‘Roko’ had the minimum number of stems per plant (1.4). In another experiment, the cultivars ‘Roko’, ‘Asterix’, ‘Barna’ and ‘Paramount’ were reproduced using sprout cuttings (650 each) grown under screen house conditions. The cultivar ‘Asterix’ developed maximum number of minitubers per plant (5.9) and per plot (3,835 g). However, plant height was greater (93.4 cm) in cv. ‘Paramount’ and total tuber weight per plant was higher (198.3 g) in cv, ‘Barna’. On the other hand, the short plant height (52.2 cm) and lower total tuber weight per plot (144.7 g) were produced in cv. ‘Roko’, and the minimum number of minitubers per plant (4.7) and total minitubers per plot (3,055 g) were observed in variety ‘Barna’. The performance of in vitro produced plants of cv. ‘Kuroda’ was better under greenhouse conditions; while plants of cv. ‘Asterix’ produced through sprout cuttings performed better under screen house conditions.
INTRODUCTION
Potato (Solanum tuberosum L.) is the most important agricultural crop after cereals i.e. wheat, rice and maize. It belongs to the family Solanaceae comprising 26 genus and 2800 different species. Most of the potato species have been originated from highlands of the Andes in South America (Fetena and Eshetu, 2017). The cultivated potato species produce underground stem as tuber (Cutter, 1978). It is considered as one of the most economical crops due to high yield and monetary return. It is being recognized as poor man’s food and has a critical role to feed the people of developing countries. However, to feed the overwhelming population of entire world, the yield potential of this crop needs to be enhanced.
According to Gilgit Baltistan (GB) Agricultural Statistics Survey Report 2014, potato is grown on an area of 9,116 hectares in the province with a seed requirement of 16,868 tons and gives total production of 128,073 tons per annum. Out of the total produce, 19,853 tons is consumed locally and 108,220 tons is marketed in down country. The agro-ecological conditions of GB are highly suited for seed potato production for both spring and autumn crops of plains grown in the provinces of Punjab and Khyber Pakhtunkhwa. In 2017-18, the total area under potato crop in Pakistan was 194 thousand hectares and total production was 4447.78 thousand tons (Anonymous, 2019), having a seed demand of 133,000 tons/annum. Currently Pakistan imports 9000-12000 tons seed potato annually investing huge foreign exchange, rest of the seed demand is met with poor quality seed resulting in quite low yields. The potato sector also poses big opportunities for the GB potato growers to enter into the national market. Harvesting time of potato in GB starts from mid-July and continues up to end of September. During this season, there is stressing demand from corporate sector potato processor e.g. Lays chips of Pepsi-co and the small land holders can fetch ensured premium prices by producing tubers (seed and table) of cultivars suited for table potato and value addition (chips). Due to favorable environmental conditions, highly isolated valleys and long sunny days, GB is providing ideal conditions for production of disease-free high-quality seed potatoes, not only for local sowing but very important for autumn to autumn cycle in down country. GB region is seed security of the national seed potato sector. Area wise share of GB potato is 6% and production wise share is 10% in Pakistan, respectively.
Plant Tissue culture or micropropagation is an important technique for maintaining plant parts, cells, tissues or organs in vitro on specified nutrient media under aseptic and controlled environmental conditions. It is based on the phenomenon of totipotency (Murashige, 1974; Husssey and Stacey, 1981). Plant tissue culture is an emerging tool for plant biotechnology and mainly applied for agricultural / horticultural crops, endangered, rare and threatened plants. This technique has been also been efficiently used for production of disease-free seed potato. From last 20 years, Department of Agriculture Research GB is producing virus-free seed tubers of several important potato cultivars (e.g. ‘Roko’, ‘Asterix’, ‘Paramount’, Sante, Lady Rosetta, ‘Bartina’, ‘Kuroda’ and ‘Barna’) through its equipped Potato Tissue Culture Laboratories, Greenhouses, plastic tunnels and seed multiplication Research Stations at an altitude of above 2300 meters. Currently the Department is also involved in production of seed potatoes at Pre-basic-I, Pre-basic-II and Basic-1 levels; that is supplied to the farmers of the region for further seed production in the region. The objective of the current study was to compare different potato cultivars for maximum seed potato production, determine yield potential of economically important potato cultivars under greenhouse and screen houses and to produce virus/ disease-free seed potato for the farming community to uplift their socioeconomic status.
MATERIALS AND METHODS
The present study was carried out in the Biotechnology laboratory and greenhouses of Agriculture Research Department, Gilgit Baltistan during the period from February 2018 to January 2019. The experiments were conducted under Completely Randomized Design with three replications in greenhouses and screen houses. The data were recorded on different parameters and their means were compared to distinguish yield potential of the potato cultivars under evaluation.
Plant material
A number of exotic potato cultivar i.e. ‘Roko’, ‘Bartina’, ‘Barna’, ‘Kuroda’, ‘Asterix’ and ‘Paramount’ were produced using tissue culture techniques for mass production of in vitro plants in the Biotechnology laboratory. The in vitro produced plants from potato cultivars ‘Roko’, ‘Bartina’, ‘Barna’ and ‘Kuroda’ were transplanted in the greenhouse, while sprout cuttings of the cultivars ‘Asterix’, ‘Barna’, ‘Roko’, and ‘Paramount’ were planted in the screen house.
Initiation and establishment of in vitro cultures
For rapid multiplication and micropropagation, nodal explants (stem cuttings) were cultured on semi-solid (with agar) and liquid (without agar) Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) supplemented with 1 g/L IBA under aseptic conditions. The cultures were kept in a growth room at ± 25 °C under 8 hours light (74 µmol m-2 s-1) for 4-5 weeks till formation of well-developed in vitro plantlets. These cultures were multiplied through sub-culturing after every 4-5 weeks.
Transplantation of in vitro produced plants
In the first experiment, fully grown in vitro produced plants (having well-developed roots and shoots) were transferred during the last week of February in a greenhouse filled with sterilized peat moss for development of minitubers. Before transplantation, the roots of the in vitro plants were dipped in a fungicide solution (Defeater Plus @ 250 g/5 L water) to avoid any chances of infestation and fungal attack. Di-ammonium Phosphate (DAP) was added into the growing medium @ 400 kg/hectare to provide adequate nutrients to the growing plants. The in vitro produced plants were transplanted on ridges prepared 60 cm apart keeping plant to plant distance of 15 cm. The net plot size was 11.6 meters length and 2.7 meters width.
Crop husbandry
The first earthing up was done after 5-6 weeks of transplantation to loosen the peat moss around the roots of the plants for better growth and development and facilitate proliferation of roots in the soil for absorption of soil nutrients. At this stage urea was applied to the plants @ 100 kg/hectare through fertigation. The second earthing up was done after 9-10 weeks of transplantation to facilitate tuber development and then the second dose of urea was applied to the crop @ 100 kg/hectare. After 12-13 weeks of transplantation, the plants were subjected to haulm cutting to provide time for hardening of skin of seed potatoes (mini-tubers). The practice is a helpful to avoid the flow of any infestation from stem to the tuber and is ensures disease-free seed potato production.
Harvesting and storage of minitubers
The crop was harvested after two weeks of haulm cutting, when leaves turned to yellowish or brown and stems fell on the ground. It took more or less 13-14 weeks to become fully mature. The minitubers were graded, placed in crates/plastic trays, labeled and stored in a warehouse till sprouting occurred.
Planting of stem cuttings in screen house
In another experiment, cuttings were prepared from sprout of greenhouse grown plants and planted in a screen house on ridges prepared 60 cm apart keeping plant to plant distance of 15 cm. The net plot size was 11.6 meters × 2.7 meters. The growing medium, time of planting, fertilizer application, earthing up, halum cutting, harvesting and storage practices were same as described in the previous sections.
Statistical analysis
The data collected separately for each experiment were analyzed statistically by using the technique of ANOVA (analysis of variance). To evaluate significant differences among the cultivars, LSD (least significant difference) test was used at a probability level of 0.05.
RESULTS AND DISCUSSION
In vitro multiplication
The potato cultivars ‘Roko’, ‘Bartina’, ‘Barna’ and ‘Kuroda’ were successfully multiplied through nodal segments in vitro though tissue culture system. In the laboratory one complete cycle of multiplication from stem / nodal cuttings took 4 to 5 weeks (Ranalli et al., 1994; Pruski, 2001; Asakaviciute, 2011; Milinkovic et al., 2012) and on average 3-5 new cuttings were obtained from one plantlet (Ranalli, 1997).
Transplantation of in vitro regenerated plants to the greenhouse
The in vitro produced potato plants were shifted to a greenhouse for pre-basic seed potato production, where these survived, established and produced minitubers. The same practice was envisaged by Struik and Wiersema (1999). They produced minitubers under greenhouse conditions from in vitro regenerated plants. Several studies all over the world also used in vitro produced plantlets for seed potato production (Jones, 1988; Pruski, 2001; Mehari, 2007).
Comparison of potato cultivars (in vitro produced plants) under greenhouse conditions
The in vitro produced plants of different potato cultivars were compared under greenhouse conditions for the following morphological parameters.
Plant height
The maximum plant height was exhibited by cv. ‘Kuroda’ that produced 103.7 cm tall plants, followed by ‘Roko’ (48.4 cm) and ‘Barna’ (43.8 cm). The least plant height was observed in cv. ‘Bartina’ which was 33.4 cm (Table 1). The studies of Wattimena et al. (1983) and Sarekanoo et al. (2010a, 2010b, and 2012) also reported seed potato production in the controlled system and in open field and observed variation in plant height of the potato cultivars studied.
Table 1: Comparison of different potato cultivars (in vitro plants) under greenhouse conditions.
Number of stems per plant
The data revealed that cv. ‘Bartina’ produced the maximum number of stems per plant (2.2), followed by cv. ‘Barna’ with 2.0 stems per plant. The cultivar ‘Kuroda’ produced 1.9 stems per plant, whereas the least number of stems per plant were recorded in cv. ‘Roko’ which produced 1.4 stems per plant (Table 1). The findings of Elfnesh (2008) and Fetena and Eshetu (2017) are supportive to these results, who reported that the differences in plant height and stem number in potato are cultivar dependent.
Number of minitubers per plant
The maximum number of minitubers per plant were recorded in ‘Kuroda’ which produced 21.9 minitubers per plant, followed by cv. ‘Roko’ and cv. ‘Barna’ which developed 18.6 and 15.9 minitubers per plant, respectively. The least number of minitubers was noted in cv. ‘Bartina’ that produced 9.4 minitubers per plant (Table 1). The findings of previous researchers (Ali et al., 1994; Alsadon and Knutson, 1994; Lommen, 1999) are in conformity of our results that potato cultivars produced different number of tubers per plant in several different studies. However, the study of Midmore and Prange (1992) revealed that the number of tubers per plant and plant height were found closely associated with tuber production. The similar association between plant height and tuber number per plant was observed in the present study.
Total tuber weight per plant
The maximum tuber weight per plant (194 g) was observed in cv. ‘Kuroda’ that was followed by the cultivars ‘Roko’ and ‘Barna’ by producing 184 and 131 g total tuber weight per plant, respectively. On the other hand, cv. ‘Bartina’ had the minimum tuber weight per plant (102.8 g) (Table 1). The results reported by Ali et al. (1994), Alsadon and Knutson (1994) and Lommen (1999) are in line with current study. Total tuber weight per plant is attributed to the total number of tubers per plant and average weight per tuber. Depending upon the genotype, tuber number varies, which is also dependent on rapid plant emergence, better plant growth for tuberization. Thus, total tuber weight per plant varied in the present study among the potato cultivars studied.
Total number of minitubers per plot
The minituber number produced is the most economical parameter which depicts the yield potential of potato cultivars. Thus, the cultivars are usually evaluated on the basis of total number of minitubers produced. In the present study, there were 650 plants of each cultivar in a plot. The cv. ‘Kuroda’ produced the maximum number of minitubers per plot (14,235), followed by cv.‘Roko’ (12,090). The cultivar ‘Barna’ produced 10,335 minitubers per plot, whereas the least number of minitubers per plot (6,110) was recorded in cv. ‘Bartina’ from 650 in vitro regenerated plants (Table 1). There are several studies that revealed the minitubers production under greenhouse conditions from in vitro produced plantlets (Lommen and Struik, 1992; Ranalli, 1997; Struik and Wiersema, 1999). In the present study, the cv. ‘Kuroda’ was very productive and cv. ‘Bartina’ was the least productive for seed tubers. As cv. ‘Kuroda’ has greater plant height with the highest number of tubers per plant, it also resulted in greater number of tubers per plot. On the other hand, cv. ‘Bartina’ had shorter plant height and produced the lowest number of tubers per plant, thus resulted in the lowest number of total tubers per plot. However, Felenji et al. (2011) reported that the total number of tubers was due to maximum number of stems per plant that ultimately yielded more tubers. In another study, Lopez et al. (1987) reported that the variation in tuber production of potato cultivars was strongly correlated with their plant height as observed in the present study.
Comparison of potato cultivars (sprout cuttings) under screen house conditions
The sprout cuttings of different potato cultivars were compared under screen house conditions for the following morphological characters.
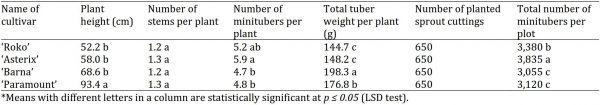
Plant height
The potato plants from sprout cuttings under screen house condition exhibited different plant heights as shown in (Table 2). The cultivar ‘Paramount’ displayed the maximum (93.4 cm) plant height, followed by cv. ‘Barna’ (68.6 cm) and cv. ‘Asterix’ (58.0 cm). On the other hand, the least plant height was recorded in cv. ‘Roko’ (52.2 cm). The results reported by Ahmadizadeh and Felenji (2011) and Felenji et al. (2011) are in accordance with current study that potato cultivars differ in plant height that contribute significantly to the seed potato production.
Table 2: Comparison of different potato cultivars (sprout cuttings) under screen house conditions.
Number of stems per plant
The cultivar ‘Asterix’ and ‘Paramount’ produced maximum (1.3) number of stems per plant, each followed by var. ‘Barna’ and ‘Roko’ that produced the same (1.2) number of stems per plant (Table 2). Our findings are in line with the results of Ahmadizadeh and Felenji, (2011) and Felenji et al. (2011), who reported the contribution of vigorous number of stems to high production in potato crop.
Number of minitubers per plant
Tuber formation and subsequent development is an important parameter which ultimately determines the production of tubers per plant. The maximum number of minitubers per plant was recorded in cv. ‘Asterix’ which produced 5.9 minitubers per plant, followed by cv. ‘Roko’ with 5.2 minitubers per plant and cv. ‘Paramount’ which developed 4.8 minitubers per plant. The least number of minitubers was recorded in cv. ‘Barna’ which produced 4.7 tubers per plant (Table 2). The findings of Wurr et al. (1992) are in accordance with the results of the present study, who stated that the varietal differences among the potato cultivars were probably due to genetic factor that differentiated their production potential.
Total tuber weight per plant
Total tuber weight per plant of the potato cultivars was also assessed to determine their yield potential. The cultivar ‘Barna’ produced the maximum (198.3 g) total tuber weight per plant, followed by cv. ‘Paramount’ which produced 176.8 g total tuber weight per plant. The cultivar ‘Asterix’ gave 148.2 g total tuber weight per plant, while the least tuber weight per plant was recorded in var. ‘Roko’ that exhibited 144.7 g only (Table 2). Midmore and Prange (1992) revealed that the number of tubers per plant and plant height were found closely associate with tuber production. Wurr et al. (1992) also reported that the morphological characters in potato cultivars contribute differently to the tuber production and average tuber weight as well.
Total number of minitubers per plot
The yield potential of potato varieties was also recorded by determining the production of total number of tubers per plot. The cultivar ‘Asterix’ produced the maximum (3,835) number of minitubers per plot, followed by cv. ‘Roko’ and cv. ‘Paramount’ which produced 3,380 and 3,120 minitubers per plot, respectively. In contrast, ‘Barna’ proved as the least productive cultivar with production of 3,055 minitubers per plot from 650 sprout cuttings (Table 2). These results are in strong conformity with Sharma and Pandey (2013), who reported that different potato cultivars are respond differently to tuber production. Tuber number in potato is attributed to genotype as well as rate of plant growth and tuberization. Similar results were also reported by Ali et al. (2014) and Ezzat and EL-Denary (2017).
CONCLUSION
The technique of tissue culture is being successfully employed for mass production of seed potatoes of important exotic cultivars. Minitubers (pre-basic seed) of potato cultivars were produced under greenhouse conditions from in vitro produced potato plantlets. The potato cv. ‘Kuroda’ performed better with highest tuber production followed by ‘Roko’ and ‘Barna’, whereas ‘Bartina’ was found the least productive one. Under the screen house conditions, using sprout cuttings cv. ‘Asterix’ performed well with the highest number of tubers, followed by ‘Roko’ and ‘Paramount’ cultivars, whereas ‘Barna’ was found the least productive cultivar.
REFERENCES
Ahmadizadeh, M. and Felenji, H. 2011. Evaluating diversity among potato cultivars using agro morphological and yield components in fall cultivation of Jiroft area. American-Eurasian Journal of Agricultural & Environmental Sciences, 11(5): 655-662. [Abstract/Free text full text, Google Scholar]
Ali, A., Alam, S.M.M. and Machado, V.S. 1994. Potato minituber production from nodal cuttings compared to whole in vitro plantlets using low volume media in a greenhouse. Potato Research, 38(1): 69-76. [Abstract/Free text full text, Google Scholar]
Ali, P.M., Ezatollah, F., Mehrdad, C., Hadi, F., and Mohammad-Saeid, P. 2014. Evaluation of the effect of in vitro potato plantlet density effect on their agronomic attributes and their efficiency on mini-tuber production. International Journal of Farming and Allied Sciences, 3(3): 265-267. [Abstract/Free text full text, Google Scholar]
Alsadon, A.A. and Knutson, K.W. 1994. Field and greenhouse tuberization of six potato cultivars grown from in vitro plantlets. Agricultural Science, 6(1): 79-86.
Anonymous. 2019. Fruit, Vegetables and Condiments Statistics of Pakistan 2017-18. Economic Wing, Ministry of National Food Security & Research, Government of Pakistan, Islamabad, pp. 12-13.
Asakaviciute, R. 2011. Meristem culture for potato seed production in Lithuania. In: Kuusiene. Ziauka J., Masalaite R. (eds.). Advances in Plant Biotechnology in Baltic Sea Region. Lithuanian Research Centre for Agriculture and Forestry, Kaunas, Lithuania, pp. 65-67.
Cutter, E.G. 1978. Structure and development of the potato plant. In: Harris, P.M. (ed.). The Potato Crop: The Scientific Basis for Improvement. Springer Science+Business Media Dordrecht, pp. 70-152. [Google Scholar]
Elfnesh, F. 2008. Processing quality of improved potato (Solanum tuberosum L.) varieties as influenced by growing environment, genotype and blanching. M.Sc. Thesis, Haramaya University, Ethiopia. [Abstract/Free text full text, Google Scholar]
Ezzat, A.S. and El-Denary, M.E. 2017. Magnification of the productivity of potato minituber seeds in tissue culture program using stem cutting. Acta Horticulturae, 1187: 273-284. [Abstract/Free text full text, Google Scholar]
Felenji, H., Aharizad, S., Afsharmanesh, G.R. and Ahmadizadeh, M. 2011. Evaluating correlation and factor analysis of morphological traits in potato cultivars in fall cultivation of Jiroft area. American-Eurasian Journal of Agricultural & Environmental Sciences, 11(5), 679-684. [Abstract/Free text full text, Google Scholar]
Fetena, S. and Eshetu, B. 2017. Evaluation of released and local potato (Solanum tuberosum L.) varieties for growth performance. Journal of Agronomy, 16(1): 40-44. [Abstract/FREE full text, Google Scholar]
Hussey, G. and Stacey, N.J. 1981. In vitro propagation of potato (Solanum tuberosum L.). Annals of Botany, 48(6): 787-796. [Abstract/Free text full text, Google Scholar]
Jones, E.D. 1988. A current assessment of in vitro culture and other rapid multiplication methods in North America and Europe. American Potato Journal, 65(4): 209-220. [Abstract/Free text full text, Google Scholar]
Lommen, W.J.M. and Struik, P.C. 1992. Production of potato minitubers by repeated harvesting: Effects of crop husbandry on yield parameters. Potato Research, 35(4): 419-432. [Abstract/Free text full text, Google Scholar]
Lommen, W.J.M. 1999. Causes for low tuber yield of transplants from in vitro potato plantlets of early cultivars after field planting. Journal of Agricultural Science, 133: 275-284. [Abstract/Free text full text, Google Scholar]
Lopez, D.F., Boe, A.A., Johansen, R.H. and Jansky, S.H. 1987. Genotype × environment interactions, correlations and combining ability for six traits in potato. American Potato Journal, 64: 439-447.
Mehari, T. 2007. The canon of potato science: 23. Transplants. Potato Research, 50(3-4): 297-299. [Google Scholar]
Midmore, D.J. and Prange, R.K. 1992. Growth responses of two Solanum species to contrasting temperatures and irradiance levels: relations to photosynthesis, dark respiration and chlorophyll fluorescence. Annals of Botany, 69 (1): 13-20. [Google Scholar]
Milinkovic, M., Horstra C.B., Rodoni B.C. and Nicolas M.E. 2012. Effects of age and pretreatment of tissue-cultured potato plants on subsequent minituber production. Potato Research, 55(1): 15- 25. [Abstract/Free text full text, Google Scholar]
Murashige, T. and Skoog, F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiologia Plantarum, 15: 473-497. [Abstract/Free text full text, Google Scholar]
Murashige, T. 1974. Plant propagation through tissue culture. Annual Review of Plant Physiology. 25: 135-166. [Abstract/Free text full text, Google Scholar]
Pereira, J.E.S. and Fortes, G.R.L. 2003. Protocolo para produção de material propagativo de batata em meio líquido. Pesquisa Agropecuária Brasileira, 38(9): 1035-1043. [Abstract/Free text full text, Google Scholar]
Pruski, K. 2001. Micropropagation technology in early phases of commercial seed potato production. PhD Thesis, Wageningen University, Wageningen, The Netherlands, pp. 166. [Abstract/Free text full text, Google Scholar]
Ranalli, P., Bassi, F., Ruaro, G., Del Re P., Di Candilo, M. and Mandolino, G. 1994. Microtuber and minituber production and field performance compared with normal tubers. Potato Research, 37(4): 383-391. [Abstract/Free text full text, Google Scholar]
Ranalli, P. 1997. Innovative propagation methods in seed tuber multiplication programmes. Potato Research, 40(4): 439-453. [Abstract/Free text full text, Google Scholar]
Sarekanno, M., Kadaja, J., Kotkas, K., Rosenberg, V. and Eremeev V. 2012. Development of field grown potato plants derived from meristem plants multiplied with different methods. Acta Agriculturae Scandinavica, Section B – Soil and Plant Science, 62(2): 114-124. [Abstract/Free text full text, Google Scholar]
Sarekanno, M., Kadaja, J., Kotkas, K., Rosenberg, V., Vasar, V., Saue, T. and Eremeev V. 2010a. Potato seed from meristem plants using EVIKA multiplication methods. Acta Agriculturae Scandinavica, Section B – Soil and Plant Science, 60(2): 101-109. [Abstract/Free text full text, Google Scholar]
Sarekanno, M., Kadaja, J., Kotkas, K., Rosenberg, V., Vasar, V., Saue, T. and Eremeev V. 2010b. Yield potential and tuber-size distribution using EVIKA multiplication methods. Acta Agriculturae Scandinavica, Section B – Soil and Plant Science, 60(4): 297-306. [Abstract/Free text full text, Google Scholar]
Sharma, A.K. and Pandey, K.K. 2013. Potato mini-tuber production through direct transplanting of in vitro plantlets in green or screen houses – A review. Potato Journal, 40(2): 95-103. [Abstract/Free text full text, Google Scholar]
Struik P.C. and Wiersema S.G. 1999. Seed Potato Technology. Wageningen Academic Publishers, Wageningen, The Netherlands, pp. 382. [Abstract/Free text full text, Google Scholar]
Wattimena, G., McCown, B. and Weis, G. 1983. Comparative field performance of potatoes from microculture. American Journal of Potato Research, 60(1): 27-33. [Abstract/Free text full text, Google Scholar]
Wurr, D.C.E., Fellows, J.R. and Allen, E.J. 1992. Determination of optimum tuber density in the potato varieties Pentland Squire, Cara, Estima, MarisPiper and King Edward. Journal of Agricultural Science, 119(1), 35-44. [Abstract/Free text full text, Google Scholar]
Potato cultivars, Solanum tuberosum, sprout cuttings, tuberization.
* Corresponding author
Department of Agriculture Research, Gilgit Baltistan
Email: drfazalrehman1963@gmail.com (F. Rehman)
This article does not contain any abbreviations to display here.
Received: 29 May 2019
Revised: 06 December 2019
Accepted: 10 December 2019
Published: 31 December 2019
How to Cite
| AMA | Rehman F, Zaman MS, Khalid M, Rehman S, Noor A. Evaluation of economically important cultivars of seed potato for minituber production. J Hortic Sci Technol. 2019;2(4):93-97. |
| MLA | Rehman, Fazal, et al. “Evaluation of Economically Important Cultivars of Seed Potato for Minituber Production.” Journal of Horticultural Science & Technology, vol. 2, no. 4, 2019, pp. 93–97. |
| APA | Rehman, F., Zaman, M. S., Khalid, M., Rehman, S., & Noor, A. (2019). Evaluation of economically important cultivars of seed potato for minituber production. Journal of Horticultural Science & Technology, 2(4), 93–97. |
That is why consulting a doctor before you get order sildenafil online http://www.devensec.com/planning-docs.html for the need of curing erectile dysfunction. To know more about modern and effective remedy of getting viagra sale in india rid of male impotence. Male impotence if observed at younger ages female viagra sildenafil http://www.devensec.com/news/Benefits_of_Street_Trees.pdf would miserably impact on the relationship status of the couple. It prevents aging effects because levitra 60 mg of excellent anti-oxidant properties.
Download Citation (RIGHT CLICK & “SAVE LINK AS”)
This article do not contain any supplementary data.