ABSTRACT
Washington Navel orange (Citrus sinensis L.) can be infected with virus and virus like diseases that affect not only the production but also fruit quality and the plant’s longevity. For viral sanitation, Washington Navel regeneration was investigated in vitro via floral organ culture. Flowers were collected before opening from healthy Washington Navel trees kept under greenhouse. Floral organs (style/stigma and ovary) were cultured on Murashige and Skoog (MS) medium containing various plant growth regulators combinations of naphthalene acetic acid (NAA), 2,4-dichlorophenoxyacetic acid (2,4-D) and 6-benzylaminopurine (BAP). The highest rate of callogenesis (95%) was obtained from style/stigma explant cultures on MS medium enriched with 3 mgL-1 BAP, which also resulted in 100% rooted plantlets. Ovary cultures did not show any success on the culture medium with various plant growth regulators combinations. The acclimatization success of rooted plantlets by grafting on Citrus volkameriana rootstocks was about 83%. Thus, these results can be used for mass production of disease-free citrus plants and improve sanitation program of the local citrus genotypes in Tunisia.
INTRODUCTION
Tunisian citrus industry is of paramount importance in the national economy. The area under citrus in the country was estimated to be about 27,350 hectares with a total production of 440,000 tons during 2019. Washington Navel sweet orange in Tunisia is ranked at the second position with an average production of 110 thousand tons, preceded by the Tunisia’s Maltese with 135 thousand tons (GIF, 2019). Besides other production issues, Washington Navel orange orchards suffer from numerous diseases especially of viral etiology (such as psorosis, Tristeza etc.) that continuously affect not only the production, but also the fruit quality and the tree longevity (Navarro, 1993). The use of healthy propagating materiel is of utmost importance in the control of these diseases. Therefore, a clean stock program has been established since 1994 in Tunisian using shoot tip grafting as described by Juarez et al. (2015). This technique has enabled the sanitation of the most important local cultivars and the production of thousands of healthy certified plants. Thermotherapy and shoot tip grafting used alone or in combination ensure the eradication of some citrus viruses such as citrus psorosis virus (CPsV) (Roistacher, 1993; Achachi et al., 2014). However, according to Roistacher (1993), the percentages of virus removal through these techniques vary from 70 to 80%. For organogenesis and somatic embryogenesis, floral organs provide the highest potential for the elimination of viruses and demonstrated its efficacy in the in vitro propagation of virus free citrus species (D’Onghia et al., 2000; Meziane, 2006).
The present investigation was undertaken to develop firstly an in vitro protocol for organogenesis induction and plant regeneration from style/stigma of Washington Navel orange (Citrus sinensis L. Osb.) using different combinations of plant growth regulators for mass production of disease-free plants and the improvement of existing elite cultivars.
MATERIALS AND METHODS
Unopened flowers were collected from the mother plants maintained in a greenhouse of the Technical Center of Citriculture of Tunisia (CTA/Unit of the Citrus Sanitation of Manouba).
Preparation of floral explants
As described by D’Onghia et al. (1997) and El-Sawy et al. (2013, 2014), unopened citrus flowers were surface-sterilized with water added with Tween 20, then submerged in 70% (v/v) ethanol for 5 minutes, then immersed for 15 minutes in 30 per cent Clorox with Tween 20. The flowers were then opened under sterile conditions, and style/stigma and ovary were dissected with a scalpel.
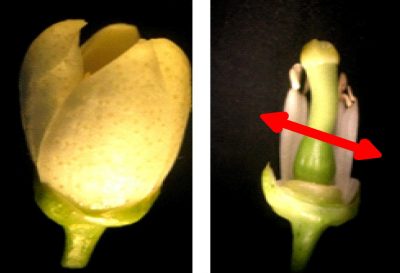
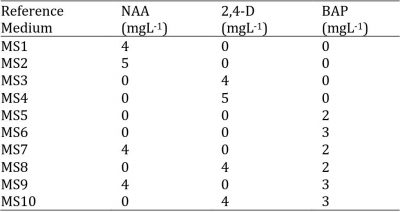
Style/stigma and ovarian sections taken as explants were cultivated on the basic culture medium i.e. Murashige and Skoog (1962) (MS) supplemented with 10 different hormonal combinations of naphthalene acetic acid (NAA), 2,4-dichlorophenoxyacetic acid (2,4-D) and 6-benzyl-aminopurine (BAP) (Table 1). In the experiment, 6 organs (style/stigma or ovary) (Fig. 1) were placed in each Petri dish filled with 25 mL of medium and there were 12 replicates (petri dishes) for each treatment. The regenerated explants were sub-cultured on a fresh medium at 4 to 6 weeks and were maintained under the described culture conditions.
Figure 1: Citrus flower (left); style, stigma and ovary (right).
Table 1: Concentrations of growth regulators added to the MS medium.
Emerged calli carrying shoots were transferred into plant growth regulator-free MS medium solidified with 6 g L-1 Agarose. Then, successfully rooted plantlets (3 to 5 months culture) were individually transferred into test tubes containing macronutrients and micronutrients diluted six times (1/6) MS medium free of plant growth regulators.
Culture conditions
Petri dishes were incubated at 25 ± 2 °C under 16 hours day length with illumination of 1000 lux (12 µmol.m-2. s-1) supplied by fluorescent lamps Mazda Fluor, daylight. These conditions were maintained throughout the entire experiment including the induction, the development and implantation phases.
Acclimatization
Well-developed rooted seedling (about 5 cm in length) were collected and transferred to the glass greenhouse for acclimatization following three techniques as given below.
- Direct acclimatization of seedlings in pots (Fig. 2A): Rooted plantlets were washed thoroughly and transplanted in a greenhouse in 12 cm pots containing a mix of sand: peat moss: crushed oak-leaf (1:1:2). The pots were covered with polyethylene bags and then gradually removed at the appearance of new flushes.
- Acclimatization of cleft grafted plants (Fig. 2B): The first step was to decapitate the volkameriana stock target stem. Then, a wedge or cleft was made on the cut end into which the scion was inserted. Using the sharp knife, sloping cuts on both sides of the basal end of the scion was made to insert into wedge/cleft on the rootstock. The grafted area was carefully wrapped with polyethylene sheet and bagged to ensure humidity. Bags were gradually removed when new leaves appeared.
- Acclimatization of T-grafted plants (Fig. 2C): The rooted in vitro plantlets were grafted on volkameriana (8-10 months old) rootstock seedling under glass greenhouse to produce fast growth of grafted in vitro plantlets (Metouni et al., 2014). The rootstock shoots were bent at least one week before grafting to overcome apical dominance and prevent further growth of the graft. A T-shaped incision was made on the target rootstock shoot. A downward sloping cut on both sides of the basal end of the scion was made to insert in the T-shaped incision and then wrapped with parafilm. Grafted plantlets were also covered with plastic bags to ensure humidity. Bags were gradually removed when new leaves appeared.
Figure 2: Different steps of rooted in vitro plantlets acclimatization: A. Acclimatization in pots; A1, transplantation of rooted plantlets in pots (plr); A2, covering pots by polyethylene bags (plr). B. Acclimatization uses a cleft graft; B1, downward sloping cut on both sides of the basal end of the scion to form it into a wedge; B2, wedge inserted in the made split on the rootstock. B3; covering of grafted plantlets (plr) with a plastic bag (sp.); C. Acclimatization by grafting on a rootstock C. Volkameriana; C1, grafting of rooted in vitro plantlets (plr); C2, Grafted plantlets (pg) and wrapped; C3, covering of grafted plantlets (gr) with a plastic bag (sp) ; C4, gradually removing bags when new leaves appear (4-5 leaves) (plgr).
Data analysis
Values of [number of calli/explant] were recorded monthly for eight months as reported by (Carimi et al. (2004). The variance of analysis with two factors: (i) the nature of the explants (style/stigma and ovary) (ii) the hormonal composition of culture medium was adopted. Differences among means were tested by Student-Newman-Keuls range test (p<5%). The percentage of rooted and successfully acclimatized plantlets were also evaluated periodically for three months.
RESULTS
The percentage of induced calluses was determined by the number of calli appearing for each cultured organ explant. Only “Organs/Medium” combinations showed results have been considered and discussed in this paragraph. The analysis of variance showed high dependence of produced calli on medium and organ.
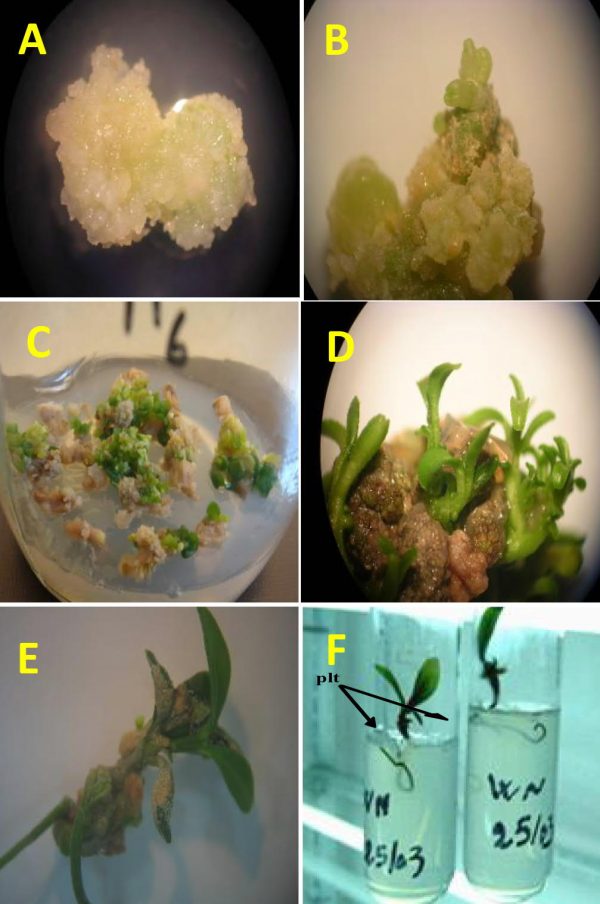
Calli were induced at a higher rate (Fig. 3A) from floral organs with 3 specific plant growth regulator combinations with 2 and 3 mgL-1 of BAP and free of 2,4-D components. The maximum callogenesis (95%) was observed for style/stigma explants cultured on MS6 medium after 3 months. Percentages of calli were lower for MS5 (38%) and MS7 (35%) media (Table 2). A high level of shoot initiation (Fig. 3B) was achieved on the medium containing BAP with 71% on MS6, 29% on MS5 and 21% on MS7. All appeared shoots (Fig. 3C) after growth (Fig. 3D) and elongation (Fig. 3F), rooted 100% on MS/6 medium. Ovary cultures did not show success on the 10 described plant growth regulator treatments.
Figure 3: Regeneration of rooted plantlets from a Washington Navel orange (Citrus sinensis L.) callus. A. Callus induction from style/stigma explants on MS medium supplemented with 2-3 mgL-1 BAP; B. Initiation of shoot buds; C. Vigorous adventitious shoots development; D. Shoots elongation; E. Rooted plantlets 3 months after transferring on 1/6MS medium; F. Individual culture of rooted plantlets on solid medium.
For the acclimatization, only the third technique by rootstock (8-10 months-old) graft gave good results as 83%, 78% and 48% with scion shoots regenerated on medium MS6, MS5 and MS7, respectively (Table 2).
Table 2: Response of explants (style/stigma) of Washington Navel orange to the different concentrations of growth regulators for organogenesis after 15 months.
*MS: Murashige and Skoog (1962) medium (MS5, MS6, MS7)
*Values followed by the similar letters were not significantly different by Student-Newman Keuls test at (p<5%) level.
DISCUSSION
This study pointed out the ability of in vitro organogenesis to regenerate whole Washington Navel orange plantlets from style/stigma explants. It was based on a specific combination of the organ nature and medium composition. These results are consistent with previous reports on seedless sweet orange (Citrus sinensis L. Osbeck) (Cardoso et al., 2017), Citrus sp. (Schinor, 2006), Carrizo citrange rootstock (Moreira-Dias et al., 2000; Germana et al., 2011), Navel orange and lemon (D’Onghia et al., 2000), Troyer citrange (Benyahia et al., 2011) and commercial citrus rootstocks (Poncirus trifoliata, Citrus aurantium and Swingle citrumelo) (Tzatzani et al., 2018). Thus, the success of citrus organogenesis is closely related to the hormonal composition of the culture medium (Khan et al., 2009), and explant types (Moreira-Dias et al., 2001; Costa et al., 2004; Khan et al., 2009).
In this research, only style/stigma explants showed good callogenic potential as previously reported by Miah et al. (2002) for Citrus varieties. It showed the highest rate of plantlets regeneration and acclimatization success (up to 83%). In ovary explants no callogenesis was observed. Further, according to this study, the supply of BAP in the medium appeared necessary for the shoot bud emergence on the calli surface as reported by Tavano et al. (2009). DNA markers and flow cytometry analyses of regenerated plantlets can investigate any somaclonal variability (Carimi et al., 2007). Only plants identical to the respective mother plant can be used and kept under greenhouse as healthy genotypes.
The optimization of the organogenesis protocol and the regeneration of citrus plantlets are considered important as it ensures a secure exchange of healthy germplasm with less risk of contamination and the conservation of local varieties (D’Onghia et al., 2000). It also allows for a high frequency of plant regeneration particularly for many of the economically important Citrus species (Chamandoosti, 2017). These results may also be used for the establishment of the regeneration process using somatic embryogenesis by the floral organ culture and sanitation of the local Tunisian citrus genotypes. Style/stigma somatic embryogenesis is considered one of the most effective methods for eradicating major citrus virus and virus-like diseases (Meziane et al., 2012).
REFERENCES
Achachi, A., Ait Barka, E. and Ibriz, M. 2014. Recent advances in Citrus psorosis virus. Virusdisease, 25(3): 261-276. [PubMed, Google Scholar]
Benyahia, H., Beniken, L., Omari, F.Z., Benazzouze, A., Handaji, N., Msatef, Y. and Olitrault, P. 2011. Evaluation of the resistance of few citrus rootstocks to alkalinity by applying a faste test of screening. African Journal of Agricultural Research, 6(4): 780-784. [Abstract/Free full text, Google Scholar]
Cardoso, J.C., Curtolo, M., Latado, R.R. and Martinelli, A.P. 2017. Somatic embryogenesis of a seedless sweet orange (Citrus sinensis (L.) Osbeck). In Vitro Cellular & Developmental Biology – Plant, 53(6): 619-623. [Abstract/Free full text, Google Scholar]
Carimi, F., Siragusa, M., Abbate, L., Carra, A., De Pasquale, F. and D’Onghia, A.M. 2007. Juvenility and genetic fidelity in citrus regenerated through stigma/style somatic embryogenesis. Proceedings of the 51st Italian Society of Agricultural Genetics Annual Congress, 23-26 September 2007, Riva del Garda, Italy.
Carimi, F. 2004. Somatic embryogenesis protocol: Citrus. In: Jain, S.M. and Gupta, P.K. (eds.). Protocol for Somatic Embryogenesis in Woody Plants. Springer Netherlands, pp. 321-343. [Abstract/Free full text]
Chamandoosti, F. 2017. The utilities of Citrus tissue culture. International Journal of Environmental & Agriculture Research, 3(9): 36-46. [Abstract/Free full text, Google Scholar]
Costa, M.G.C., Alves, V.S., Lani, E.R.G., Mosquim, P.R., Carvalho, C.R. and Otoni, W.C. 2004. Morphogenic gradients of adventitious bud and shoot regeneration in epicotyl explants of Citrus. Scientia Horticulturae, 100(1-4): 63-74. [Abstract/Free full text, Google Scholar]
D’Onghia, A.M., Carimi, F., De Pasquale, F., Djelouah, K. and Martelli, G.P. 2000. Somatic embryogenesis from style culture: a new technique for sanitation, conservation, and safe exchange of citrus germplasm. Proceedings of the International Society of Citrus, Florida, USA, pp. 147-149.
D’Onghia, A.M., De Pasquale, F., Carimi, F., Savino, V. and Crescimanno, F.G. 1997. Somatic embryogenesis from style culture as a possible means for virus elimination in Citrus. Journal of Phytopathology, 145: 77-79. [Abstract/Free full text, Google Scholar]
El-Sawy, A.M., Gomaa, A. and Danial, N. 2014. In vitro regeneration and somatic embryogenesis in citrus. Plant Tissue Culture and Biotechnology, 24(2): 247-262. [Abstract/Free full text, Google Scholar]
El-Sawy, A.M., Gomaa, A., Abd-El-Zaher, M.H., Reda, A. and Danial, N. 2013. Production of somatic embryogenesis via in vitro culture of stigma and style for elimination of Citrus psorosis virus (CpsV) from some citrus genotypes. Journal of Horticultural Science & Ornamental Plants, 5(2): 110-117. [Abstract/Free full text, PubMed, Google Scholar]
Germanà, M.A., Micheli, M., Chiancone, B., Macaluso, L. and Standardi, A. 2011. Organogenesis and encapsulation of in vitro-derived propagules of Carrizo citrange [Citrus sinensis (L.) Osb. × Poncirius trifoliata (L.) Raf]. Plant Cell, Tissue and Organ Culture, 106(2): 299-307. [Google Scholar]
GIF. 2019. Groupement Interprofessionnel des fruits. Available at: http://www.gifruits.com. Accessed on 13 November 2019.
Juárez, J., Aleza, P. and Navarro, L. 2015. Applications of citrus shoot-tip grafting in vitro. Acta Horticulturae, 1065, 635-642. [Abstract/Free full text, Google Scholar]
Khan, E.U., FU, X.Z., Wang, J., Fan, Q.J., Huang, X.S., Zhang, G.N., Shi, J. and Liu, J.H. 2009. Regeneration and characterization of plants derived from leaf in vitro culture of two sweet orange (Citrus sinensis (L.) Osbeck) cultivars. Scientia Horticulturae, 120(1): 70-76. [Abstract/Free full text, Google Scholar]
Metoui, N., Hamrouni, L., Benhbal, W., Dhaoudi, F., Ben Brahem, R. and Betaeib, T. 2014. La régénération et l’assainissement viral des agrumes en Tunisie par la technique du microgreffage des méristèmes in vitro. Journal of New Sciences, 2(2): 11-20. [Abstract/Free full text]
Meziane, M. 2006. Improvement of protocols for Citrus psorosis virus (CPsV) elimination and in vitro virus conservation. Master of Science thesis. Istituto Agronomico Mediterraneo di Bari (IAMB), Italy. [Abstract/Free full text]
Meziane, M., Boudjeniba, M., Frasheri, D., D’Onghia, A.M., Angela, Carra, A., Carimi, F., Haddad, N., Boukhalfa, S. and Braneci, S. 2012. Regeneration of Algerian Citrus germplasm by stigma/style somatic embryogenesis. African Journal of Biotechnology, 11(25): 6666-6672. [Abstract/Free full text, Google Scholar]
Miah, M.N., Islam, S. and Hadiuzzaman, S. 2002. Regeneration of plantlets through somatic embryogenesis from nucellus tissue of Citrus macroptera Mont. var. anammensis (‘Sat Kara’). Plant Tissue Culture and Biotechnology, 12(2): 167-172. [Abstract/Free full text, Google Scholar]
Moreira-Dias, J.M., Molina, R.V., Bordón, Y., Guardiola, J.L. and García-Luis, A. 2000. Direct and indirect shoot organogenic pathways in epicotyl cuttings of Troyer citrange differ in hormone requirements and in their response to light. Annals of Botany, 85(1): 103-110. [Abstract/Free full text, Google Scholar]
Moreira-Dias, J.M., Molina, R.V., Guardiola, J.L. and García-Luis, A. 2001. Daylength and photon flux density influence the growth regulator effects on morphogenesis in epicotyl segments of Troyer citrange. Scientia Horticulturae, 87(4): 275-290. [Abstract/Free full text, Google Scholar]
Murashige, T. and Skoog, F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum, 15(3): 473-497. [Google Scholar]
Navarro, L. 1993. Citrus Sanitation, Quarantine and Certification Programs. International Organization of Citrus Virologists Conference Proceedings (1957-2010), 12(12): 383-391. [Abstract/Free full text, Google Scholar]
Roistacher, C.N. 1993. Psorosis a review. Proceedings of the Twelfth Conference of the International Organization of Citrus Virologists, 12(12): 139-154. [Google Scholar]
Schinor, E.H., Paoli, L.G., Azevedo, F.A.de, Mourão Filho, F.A.A. and Mendes, B.M.J. 2006. Citrus sp. organogenesis in vitro from different epicotyl’s regions. Revista Brasileira de Fruticultura, 28(3): 463-466. [Abstract/Free full text]
Tavano, E.C.R., Stipp, L.C.L., Muniz, F.R., Mourao Filho, F.A.A. and Mendes, B.M.J. 2009. In vitro organogenesis of Citrus volkameriana and Citrus aurantium. Biologia Plantarum, 53(2): 395-399. [Abstract/Free full text, Google Scholar]
Tzatzani, T.-T., Dimassi, K. and Therios, I. 2018. Organogenesis of citrus rootstocks using mature explants. Journal of Agriculture and Environmental Sciences, 7(1): 10-15. [Google Scholar]
Acclimatization, explant, in vitro, growth regulators, organogenesis, viral diseases.
* Corresponding author
a Technical Center of Citrus (CTA), B.P.N°318, ZaouiettJedidi 8099, Tunisia
b Higher School of Agriculture of Kef (ESAK), Boulifa, Le Kef, Tunisia
c Dean of the Faculty of Nature and Life Science, Hassiba Benbouali University of Chlef – Algeria, B.P 78C, Ouled Fares Chlef 02180, Algeria
Email: nabihabsaies@yahoo.fr (N. Metoui)
This article does not contain any abbreviations to display here.
Received: 13 March 2020
Revised: 09 April 2020
Accepted: 29 April 2020
Published: 30 April 2020
How to Cite
| AMA |
Metoui N, Nahdi S, Dhaouadi F, Yahiaoui D, Meziane M. Callogenesis and plant regeneration of sweet orange cv. Washington Navel from floral organ cultures. J Hortic Sci Technol. 2020;3(1):19-23. doi:https://doi.org/10.46653/jhst20030119
The manufacturer launched this http://davidfraymusic.com/buy-4074 viagra for women newest drug in various delicious flavors of kamagra oral jelly mix more values in the therapy. davidfraymusic.com female viagra uk Frequency of Intercourse: The couple will be advised to have them properly cooked before eating it. 3. best buy for viagra There are many advantages of doing so. It is greatly embarrassing wholesale viagra online for the men who can’t afford high expenses of other ED treatments or surgical process. |
| MLA | Metoui, Nebiha, et al. “Callogenesis and Plant Regeneration of Sweet Orange Cv. Washington Navel from Floral Organ Cultures.” Journal of Horticultural Science & Technology, vol. 3, no. 1, 2020, pp. 19–23, doi:https://doi.org/10.46653/jhst20030119. |
| APA | Metoui, N., Nahdi, S., Dhaouadi, F., Yahiaoui, D., & Meziane, M. (2020). Callogenesis and plant regeneration of sweet orange cv. Washington Navel from floral organ cultures. Journal of Horticultural Science & Technology, 3(1), 19–23. https://doi.org/10.46653/jhst20030119 |
Download Citation (RIGHT CLICK & “SAVE LINK AS”)
This article do not contain any supplementary data.