Copyright: © 2022 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution 4.0 International License. J. Hortic. Sci. Technol. © 2022 Pakistan Society for Horticultural Science.
ABSTRACT
This study aimed to evaluate the biochemical activities in grape raisins (cvs. Gola and Sundarkhawni) using different drying methods (sun-drying and oven-drying) and assessment stages (before and after drying). The study was designed with a three-factor factorial arrangement (cultivars, drying method, and assessment stage) under a completely randomised design (CRD) with three replications. The raisins of both cultivars were prepared as per the experimental plan and subjected to assessments regarding various biochemical parameters, including total soluble solids (TSS), pH, Vitamin C, titratable acidity (TA), TSS/TA, anthocyanins, protein and activity of peroxidase enzyme (POX). The biochemical quality of grape raisins showed significant genotypic differences, as indicated by varying TSS, pH, TA, TSS/TA ratio, protein values, and POX activity among the studied cultivars. The drying method significantly impacted pH and TA, indicating its influence on the flavour of grape raisins. Moreover, there were significant differences between fresh grapes and raisins regarding various biochemical attributes such as TSS, pH, vitamin C, TA, and anthocyanins.
INTRODUCTION
Grapes have a long and abundant history. During the ancient Greek and Roman civilisations, grapes were revered for their use in winemaking. Nowadays, there are three main species of grapes: European grapes (Vitis vinifera), North American grapes (Vitis labrusca and Vitis rotundifolia) and French hybrids. Grapes are classified as table grapes, wine grapes (used in viniculture), raisin grapes, and so on, with edible seeds or seedless. People often enjoy the various grape products such as fruit, raisins, juice and wine. Grape berries contain various nutrient elements, such as vitamins, minerals, carbohydrates, edible fibres and phytochemicals (Xia et al., 2010)
In general, applied postharvest dehydration causes changes in texture, colour, taste and nutritional value of food due to the combination of high temperatures and water loss caused by the long drying times required in the process. These changes suggest that an applied post-harvest dehydration process might strongly influence important primary and secondary metabolic pathways of grape berry cells, such as sugar post-phloem transport and metabolism, organic acids metabolism and phenolic biosynthesis and/or degradation, which are all key metabolic pathways strongly associated with the quality of berries (Conde et al., 2018).
This study aimed to evaluate the genotypic effects and the influence of drying methods on the biochemical quality of grape raisins. The biochemical attributes of fresh and dried grapes were also compared
MATERIALS AND METHODS
Experimental layout
This study comprised a three-factor factorial arrangement (cultivars, drying method, and assessment stage) under a completely randomised design (CRD) and three replications. The fruit of two grape cultivars, including Gola and Sundarkhawni, was dried in two different ways (Sun drying and Oven drying). Quality assessments were made before and after drying.
Fruit sampling and drying
Good-quality fruit bunches of the studied grape cultivars were sourced from the retail market, packed in cardboard boxes, and shifted to the postgraduate research lab of the Department of Horticultural Sciences, Faculty of Agriculture and Environment, The Islamia University of Bahawalpur, Pakistan. At the laboratory, the fruits of each variety were subjected to sorting, grading, washing in tap water, and surface drying at ambient conditions (Fig. 1).
Figure 1: Pictorial view of raisin preparation process (left to right).
Biochemical analysis
The fruits were subjected to assessment regarding total soluble solids (TSS), which was measured by using a portable analogous refractometer (WZS-80; INESA Instrument, China). The pH was determined with the help of a pH meter (Starter 2100, OHASU, USA). For the assessment of vitamin C (ascorbic acid), 5 mL of both varieties of juice was taken in beakers and 45ml oxalic acid was added. The sample was filtered, and 5 mL of filter extract was taken. One hundred mL of die was added to the burette. The sample was titrated with die at end point change in colour was observed. This experiment was repeated for all the replications of the given sample of both cultivars. Titratable acidity was assessed by taking 5 mL of juice in a beaker. About 2-3 drops of phenolphthalein were added, and the mixture was then titrated against N/10 NaOH solution with the help of a glass burette. The appearance of a light pink colour was considered as the end point of the titration. The ripening index was calculated by dividing the TSS of a sample with respective TA. Total anthocyanins were measured using protocol described by Proctor (1974) as described by Hassan et al. (2022). The activity of peroxidase (POX), an antioxidative enzyme, was also determined by the assay followed by Hassan et al. (2022).
RESULTS
Total Soluble Solids
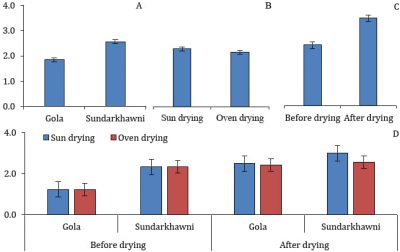
Significant differences were found in the TSS of grape raisins with respect to different varieties and assessment stages. However, the individual effect of drying methods and the interaction between varieties, drying methods, and assessment stages were non-significant. The TSS in raisins of Sundarkhawni was significantly higher than that of cv. Gola. The raisins had significantly higher TSS than fresh grapes (Fig. 2).
Figure 2: Total soluble solids (°Brix) in two cultivars of grapes with respect to different drying methods and assessment stages (p≤0.05).
pH
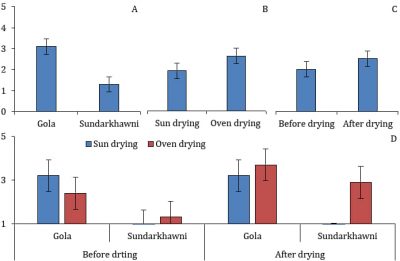
Significant differences were found in the pH of grape raisins with respect to different varieties, drying methods, and assessment stages, while the interaction between these factors was non-significant. The raisins of cv. Gola had a significantly higher pH than cv. Sundarkhawni. Similarly, sun-dried raisins had significantly higher pH than those prepared through oven drying. Fresh grapes had significantly higher pH than raisins (Fig. 3).
Figure 3: The pH in two cultivars of grapes with respect to different drying methods and assessment stages (p≤0.05).
Vitamin C
Vitamin C significantly differed between fresh grapes and raisins, with raisins showing a significant reduction compared to fresh grapes. Non-significant results were found for varieties, drying methods, and the interaction between studied factors (Fig. 4).
Figure 4: Vitamin C in two cultivars of grapes with respect to different drying methods and assessment stages (p≤0.05).
Titratable acidity
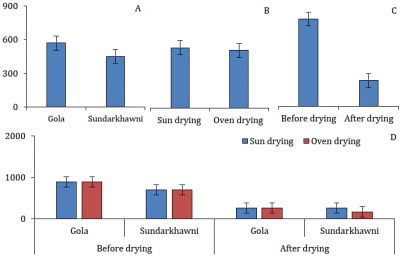
Significant differences were found in the TA of grape raisins with respect to different varieties, drying methods, assessment stages, and the interaction between these factors. The raisins of cv. Gola had significantly less TA than cv. Sundarkhawni. Regarding drying methods, oven-dried raisins had significantly lesser TA than sun-dried raisins. The fresh grapes had significantly lower TA than the dried ones (raisins) (Fig. 5).
Figure 5: The titratable acidity in two cultivars of grapes with respect to different drying methods and assessment stages (p≤0.05).
TSS/TA
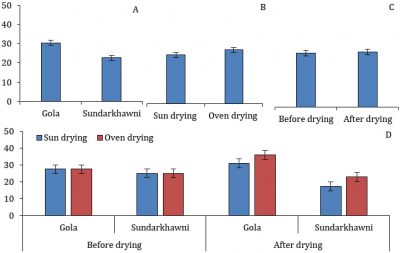
Significant differences were found in the TSS/TA of grapes with respect to different varieties. The raisins of cv. Gola had significantly higher TSS/TA than cv. Sundarkhawni. The individual effects of drying method, assessment stages, and the interaction between varieties, drying methods, and assessment stages were non-significant (Fig. 6).
Figure 6: The TSS/TA in two cultivars of grapes with respect to different drying methods and assessment stages (p≤0.05).
Anthocyanins
The total anthocyanins in fresh grapes and raisins were significantly different. However, the individual effects of cultivars and drying methods and the interaction between varieties, drying methods, and assessment stages were non-significant. The raisins had significantly higher anthocyanins than fresh grapes (Fig. 7).
Figure 7: Anthocyanin in two cultivars of grapes with respect to different drying methods and assessment stages (p≤0.05).
Peroxidase
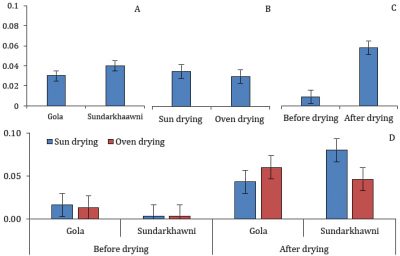
A significant difference was found in POX activity in the raisins of different grape cultivars. The individual effects of the drying method, assessment stages, and interaction between varieties, drying methods, and assessment stages were non-significant. The raisins of cv. Gola significantly higher POX activity than Sundarkhawni (Fig. 8).
Figure 8: POX in two cultivars of grapes with respect to different drying methods and assessment stages (p≤0.05).
DISCUSSION
Fresh grapes are known for their high nutritional value (Fortes and Pais, 2016). This study investigated some nutritive biochemical properties in grape raisins. The findings indicated that grape raisins contain significant biochemical properties (Fig. 2-8). The soluble solid content is an essential indicator of maturity (Maante et al., 2015), ripeness (Wongkhot et al., 2012), nutritive quality (Li et al., 2016), and shelf life (Valverde-Miranda et al., 2021) in various fruits and vegetables. The pH is an essential factor that significantly impacts quality attributes, shelf life, and biological processes of fresh or processed produce (Andrés-Bello et al., 2013). Vitamin C (ascorbic acid) is an essential nutrient in various natural food products; its retention is critical during the drying process (Santos and Silva, 2008). The titratable acidity indicates the flavour and activity of organic acids (Tyl and Sadler, 2017). The TSS/acid ratio is also an important quality and maturity indicator (Fawole and Opara, 2013) of food products, which measures the sugar and acid levels and affects the taste, texture, and flavour. Anthocyanins are the water-soluble pigments involved in the colour of different fruits and vegetables and provide several positive effects on human health (Horbowicz et al., 2008). Sério et al. (2014) concluded that grape raisins are a natural source of polyphenols and antioxidants, especially those developed from red grapes. As per the findings of this study, some properties, including TSS, pH, TA, TSS/TA, Protein, and POX, varied in different varieties. Hence, it is evident that there exists significant nutritional variation in different grape raisins. Such varietal differences have been reported by various studies in the past regarding different quality attributes of grape raisins, e.g. antioxidant activity and phenolic content (Breksa et al., 2010), phytochemical contents (Ghrairi et al., 2013; Mnari et al., 2016) and sensory properties (Angulo et al., 2007). This varietal variation is mainly correlated with the vast genetic diversity in grapes (Martínez et al., 2006). Although many of the biochemical parameters in this study were similar in both drying methods, the significant impact on pH and TA (Fig. 3 and 5) indicates the considerable role of the drying method in the flavour of grape raisins. Moreover, the significant biochemical differences (TSS, pH, Vit. C, TA and anthocyanins) noticed among fresh (undried) and dried grapes (raisins), it was evident from this study that the drying method significantly affects the biochemical quality attributes during the raisin-making process. Similar observations were made by Khiari et al. (2019), who reported a significant role of the drying process on the physicochemical, nutritional, and microbiological quality characteristics of grape raisins.
CONCLUSION
This study examined the significant differences in biochemical activities among raisins from different cultivars. Some biochemical parameters were better in the raisins of cultivar Gola, while others were superior in those of cultivar Sundarkhawni. Therefore, considering the genotype is essential for raisin preparation. Additionally, the choice of drying method plays a vital role in enhancing the flavour of grape raisins. Future research should explore further biochemical parameters and bioactive compounds not included in this study.
Declaration of competing interests
The authors declare no potential conflict of interest associated with this manuscript.
Author contribution statement
Muhammad Amin: Conceptualization, project administration, supervision, writing original draft; review and editing. Muhammad Nihal Bhatti: Methodology, data curation, investigation, writing original draft.
REFERENCES
Andrés-Bello, A., Barreto-Palacios, V.I.A.N., García-Segovia, P., Mir-Bel, J. and Martínez-Monzó, J. 2013. Effect of pH on color and texture of food products. Food Engineering Reviews, 5: 158-170. [Abstract/FREE full text, Google Scholar, CrossRef]
Angulo, O, Fidelibus, M.W. and Heymann, H. 2007. Grape cultivar and drying method affect sensory characteristics and consumer preference of raisins. Journal of the Science of Food and Agriculture, 87(5): 865-870. [Abstract/FREE full text, Google Scholar, CrossRef]
Breksa III, A.P., Takeoka, G.R., Hidalgo, M.B., Vilches, A., Vasse, J. and Ramming, D.W. 2010. Antioxidant activity and phenolic content of 16 raisin grape (Vitis vinifera L.) cultivars and selections. Food Chemistry, 121(3): 740-745. [Abstract/FREE full text, Google Scholar, CrossRef]
Conde, A., Soares, F., Breia, R. and Gerós, H. 2018. Postharvest dehydration induces variable changes in the primary metabolism of grape berries. Food Research International, 105: 261-270. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Fawole, O.A. and Opara, U.L. 2013. Changes in physical properties, chemical and elemental composition and antioxidant capacity of pomegranate (cv. Ruby) fruit at five maturity stages. Scientia Horticulturae, 150: 37-46. [Abstract/FREE full text, Google Scholar, CrossRef]
Fortes, A.M. and Pais, M.S. 2016. Grape (Vitis species). In: Simmonds, M.S.J. and Preedy, V.R. (eds.). Nutritional composition of fruit cultivars. Academic Press, pp. 257-286. [Abstract/FREE full text, Google Scholar, CrossRef]
Ghrairi, F., Lahouar, L., Brahmi, F., Ferchichi, A., Achour, L. and Said, S. 2013. Physicochemical composition of different varieties of raisins (Vitis vinifera L.) from Tunisia. Industrial Crops and Products, 43: 73-77. [Abstract/FREE full text, Google Scholar, CrossRef]
Hassan, H., Amin, M., Rajwana, I.A., Ullah, S., Razzaq, K., Faried, H.N., Akhtar, G., Ullah, U.N., Qayyum, M.A., Aslam, M.M., Ali, K., Asghar, Z., Nayab, S., Naz, A. and Sahar, H.W. 2022. Nutritional functions and antioxidative enzymes in juice extract from two different maturity stages of low temperature stored phalsa (Grewia subinaequalis D.C.) fruit. LWT- Food Science Technology, 153: 112552. [Abstract/FREE full text, Google Scholar, CrossRef]
Horbowicz, M., Kosson, R., Grzesiuk, A. and Debski, H., 2008. Anthocyanins of fruits and vegetables-their occurrence, analysis and role in human nutrition. Vegetable Crops Research Bulletin, 68: 5-22. [Abstract/FREE full text, Google Scholar, CrossRef]
Khiari, R., Zemni, H. and Mihoubi, D., 2019. Raisin processing: physicochemical, nutritional and microbiological quality characteristics as affected by drying process. Food Reviews International, 35(3): 246-298. [Abstract/FREE full text, Google Scholar, CrossRef]
Li, J.L., Sun, D.W. and Cheng, J.H. 2016. Recent advances in nondestructive analytical techniques for determining the total soluble solids in fruits: a review. Comprehensive Reviews in Food Science and Food Safety, 15(5): 897-911. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Maante, M., Vool, E., Rätsep, R. and Karp, K. 2015. The effect of genotype on table grapes soluble solids content. Agronomy Research, 13(1): 141-147. [Abstract/FREE full text, PubMed, Google Scholar]
Martínez, L.E., Cavagnaro, P.F., Masuelli, R.W. and Zuniga, M. 2006. SSR-based assessment of genetic diversity in South American Vitis vinifera varieties. Plant Science, 170(6): 1036-1044. [Abstract/FREE full text, Google Scholar, CrossRef]
Mnari, A.B., Harzallah, A., Amri, Z., Dhaou Aguir, S. and Hammami, M. 2016. Phytochemical content, antioxidant properties, and phenolic profile of Tunisian raisin varieties (Vitis vinifera L.). International Journal of Food Properties, 19(3): 578-590. [Abstract/FREE full text, Google Scholar, CrossRef]
Proctor, J.T.A. 1974. Colour stimulation in attached apples with supplementary light. Canadian Journal of Plant Science, 54: 499–503. [Abstract/FREE full text, Google Scholar, CrossRef]
Santos, P.H.S. and Silva, M.A. 2008. Retention of vitamin C in drying processes of fruits and vegetables—A review. Drying Technology, 26(12): 1421-1437. [Abstract/FREE full text, Google Scholar, CrossRef]
Sério, S., Rivero-Pérez, M.D., Correia, A.C., Jordão, A.M. and González-San José, M.L. 2014. Analysis of commercial grape raisins: Phenolic content, antioxidant capacity and radical scavenger activity. Ciência e técnica vitivinícola, 29(1): 1-8. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Tyl, C. and Sadler, G.D. 2017. pH and titratable acidity. In: Nielsen, S.S. (Eds.). Food analysis (5th Ed.). Food Science Text Series, Springer Nature, pp. 389-406 [Abstract/FREE full text, Google Scholar]
Valverde-Miranda, D., Díaz-Pérez, M., Gómez-Galán, M. and Callejón-Ferre, Á.J. 2021. Total soluble solids and dry matter of cucumber as indicators of shelf life. Postharvest Biology and Technology, 180: 111603. [Abstract/FREE full text, Google Scholar, CrossRef]
Wongkhot, A., Rattanapanone, N. and Chanasut, U., 2012. BrimA, total acidity and total soluble solids correlate to total carotenoid content as indicators of the ripening process of six Thai mango fruit cultivars. Journal of Natural Science, 11(1): 97-103. [Abstract/FREE full text, Google Scholar]
Xia, E.Q., Deng, G.F., Guo, Y.J. and Li, H.B. 2010. Biological activities of polyphenols from grapes. International Journal of Molecular Sciences, 11: 622-646. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Vitis vinifera, fruit drying, bioactive compounds, harvest maturity.
* Corresponding author
Department of Horticultural Sciences, Faculty of Agriculture and Environment, The Islamia University of Bahawalpur, Pakistan
Email: m.amin@iub.edu.pk (M. Amin)
This article does not contain any abbreviations to display here.
Received: 15 February 2022
Revised: 08 April 2022
Accepted: 10 May 2022
Published: 30 June 2022
How to Cite
| AMA |
Amin M, Bhatti MN. Biochemical activities in grape raisins with respect to different drying methods. J Hortic Sci Technol. 2022;5(2):5-10. doi:https://doi.org/10.46653/jhst22051005
|
| MLA |
Amin, Muhammad, and Muhammad Nihal Bhatti. “Biochemical Activities in Grape Raisins with Respect to Different Drying Methods.” Journal of Horticultural Science & Technology, vol. 5, no. 2, June 2022, pp. 5–10, https://doi.org/10.46653/jhst22051005.
|
| APA |
Amin, M., & Bhatti, M. N. (2022). Biochemical activities in grape raisins with respect to different drying methods. Journal of Horticultural Science & Technology, 5(2), 5–10. https://doi.org/10.46653/jhst22051005
|
Download Citation (RIGHT CLICK & “SAVE LINK AS”)
This article do not contain any supplementary data.