ABSTRACT
Delicate fruit of strawberry is susceptible to high temperature stress and fungal infection. An extensive spray program is usually adapted to secure yield and fruit quality which sometimes pose a serious threat to consumer health. However, development of eco-friendly, economical and safer strategies has always been in focus of R&D sector. In this study, field-grown strawberry plants cv. Chandler were sprayed with 1, 2 or 3 mM oxalic acid at flowering stage. Interestingly, foliar application of oxalic acid in low doses (1 mM and 2 mM) had more growth-promoting effect on strawberries whereas foliar application of 3 mM oxalic acid either negatively affected or remained ineffective. Low-dose applications of oxalic acid resulted in enhanced nitrogen (1.5-fold), phosphorus (2.5-fold) and potassium (1.75-fold) levels in leaf petioles. Increase in primary macronutrients was also correlated well with enhancement in plant growth indicators including dry biomass (1.5-fold), leaf area (1.7-fold), specific leaf area (2.8-fold) and leaf area ratio (2.6-fold), root weight ratio (1.9-fold), root-to-shoot ratio (1.4-fold). Only, leaf chlorophyll and fresh fruit weight were negatively impacted by oxalic acid. In addition to increase in number of fruits per plant, oxalic acid also improved sensory properties of strawberry fruits mainly due to increase in sugar: acid ratio (1.6-fold), ascorbic acid contents (1.2-fold) and non-reducing sugars (2-fold). Overall, foliar application of 1 mM oxalic acid favoured vegetative growth and enhanced yield and fruit quality of strawberry cv. Chandler.
INTRODUCTION
Strawberry (Fragaria × ananassa Duch.) is a nutrition-rich fruit with its global production increasing annually (Zheng et al, 2007). Currently grown commercial cultivars are mostly polyploids, as much as decaploid, and thus have fruit size larger than their predecessors (Ahmadi and Bringhurst, 1992). Strawberry cultivars are generally categorized into June bearing, ever bearing and day-neutral cultivars depending upon their requirement of day length (Hancock et al., 2017). Its fruit is very well applauded for its scrumptious taste, sweetness, colour and nutritional qualities (Anwar et al., 2017). It is rich source of phenolics, flavonoids, ascorbic acid and health-promoting natural antioxidants (Robards et al., 1999; Wang and Lin, 2000; Hansawasdi et al., 2006; Laugale and Bite, 2006; Buendia et al., 2010; Schwieterman et al., 2014; Liu et al., 2016). Thus, consumption of strawberry fruit in daily diet is crucial to scavenge reactive oxygen species and reducing occurrence possibilities of degenerative diseases (Hung et al., 2004; Seeram, 2008). Strawberry is a highly perishable commodity and has short shelf life under ambient conditions mainly because of moisture loss and fast decline towards cellular
degradation. Being skinless and delicate in nature, its quality is also negatively affected by microbial infection, mechanical damage and insect attack. So, proper production and postharvest management is imperative to tackle yield and quality-related challenges. In Pakistan, strawberry is mostly cultivated in Charsadda, Swat, Mardan, Haripur, Islamabad and Gujrat. Strawberry cv. Chandler is the only grown cultivar and from short-day requiring June-bearing class (Ahmad et al., 2018) in Pakistan. Strawberry production has emerged as fast grooming in the country in recent years. A significant share of total production cost in invested in multiple and frequent sprays of pesticides and nutrients to increase farm-gate income by improving yield and fruit quality. Residual effects from inappropriate use of these chemicals has encouraged to explore less-hazardous and economical options.
Biomass accumulation during plant growth is regulated by the uptake of minerals and nutrients from soil rhizosphere. Nutrient deficiency negatively affects metabolic changes in plants which decreases photosynthetic efficiency leading to reduced growth rate. Use of organic acids has not only been suggested to increase plant resistance against nutrient deficient environment but these have also been implicated in regulating physiological processes of plants including decrease in lipid peroxidation, improve antioxidant enzyme activity and increase in osmotic regulation (Miura and Tada, 2014). Oxalic acid, a naturally occurring organic acid, maintains membrane integrity and delay fruit ripening (Zheng et al., 2007a; Dhaiya et al., 2010). Postharvest application of oxalic acid has been shown to delay ripening and maintain postharvest quality of tomato, peach, plum, mango, pomegranate, sweet cherry and banana fruits by retarding ethylene production, respiration and production of active oxygen species, thus enhance antioxidant potential (Zheng et al., 2007a; Wang et al., 2009; Sayyari et al., 2010; Valero et al., 2011; Wu et al., 2011; Zheng et al., 2012a; Huang et al., 2013a; Huang et al., 2013b; Kant et al., 2013; Razzaq et al., 2015). Moreover, postharvest use of oxalic acid has also been appreciated with its potential to alleviate chilling injury in peach, mango and tomato (Ding et al., 2007; Lin et al., 2014; Li et al., 2014 Li et al., 2016; Razzaq et al., 2017), control browning in litchi (Zheng and Tian, 2006) and reduce decay incidence in jujube and mango (Zheng et al, 2007; Wang et al., 2009). Though, preharvest application of oxalic acid has also been reported to increase yield and/or improve nutritional quality of sweet cherry (Martinez-Espla et al., 2014), peach (Razavi and Hajilou, 2016) , plum (Martinez-Espla et al., 2018) and kiwifruit (Zhu et al., 2016) but its effect on strawberry has not yet been investigated. It is one of the major organic acids found in strawberries (Koyuncu and Dilmacunal, 2010; Liu et al., 2016). So, with an objective to explore environment-friendly and economical alternatives for strawberries, field performance of strawberry plants foliar supplied with oxalic acid were evaluated for plant growth, yield and fruit quality characteristics. Findings suggested positive effect of foliar application of oxalic acid on growth and fruit quality of strawberry plants.
MATERIALS AND METHODS
Runners of strawberry cv. Chandler of same crown size were purchased from a commercial nursery in Mingora, District Swat and transplanted on 22 cm raised ridges at 20 cm plant to plant distance under subtropical agro-climatic conditions of Faisalabad, Pakistan. After 90 days of transplantation, healthy strawberry plants were selected and marked for different treatments under randomized complete block design (RCBD). Foliar application of 0 (control), 1, 2 or 3 mM oxalic acid started 100 days after transplantation and repeated every week until the final fruit harvest.
Leaf petiole samples were collected initially a week before start of oxalic acid application and then at final fruit harvest. Nitrogen (N), phosphorus (P) and potassium (K) levels in leaf petioles were determined as described earlier by Estefan et al. (2013). At the time of final fruit harvest, relative chlorophyll content (SPAD value) in intact leaves was measured with chlorophyll meter (CCM-200 plus, Opti-Sciences, Hudson, NH, USA). After the final fruit harvest, whole plant was taken out and separated into leaves, roots and crown parts. Leaf area was measured with automated digital image analysing software package ‘Easy Leaf Area’ version 1.02 (Ealson and Bloom, 2014). After recording fresh weight, each plant section was individually packed in perforated paper bags and kept at 70 °C until constant dry weight was achieved (Kuisma et al., 2014). Plant dry weight was then calculated and presented as percent of fresh weight. Specific leaf area (leaf area divided by dry weight of leaves), leaf area ratio (leaf area divided by dry weight of whole plant), root-to-whole plant weight ratio (dry weight of roots to plant dry weight) and root-to-shoot ratio (sum of dry weight of crown and roots to dry weight of above crown parts) were calculated at final harvest according to method described by Fernandez et al. (2002). Number of flowers, fruit set and ripe fruits per plant were recorded every week from 103 days after transplantation. Harvest index was calculated by dividing dry weight of fruits over dry weight of whole plant (Martinez-Ferrri et al., 2016).
Healthy strawberries without any sign of disease, damage or bruise were randomly harvested and immediately brought to laboratory for physical, biochemical and sensory analyses. Fresh weight of 10 fruits was individually taken and averaged per replication. Then, their peduncles and calyx were removed, and edible tissues were chopped and blended. Homogenized mixture was then centrifuged at 10,000 rpm for 15 min. Supernatant was used for further biochemical analyses. Total soluble solids (TSS) and pH were determined with pH meter (HI-98107, Hanna Instruments, Mauritius) and digital hand-held refractometer (RS-5000, Atago, Japan), respectively. Total titratable acidity (TTA) was measured with titrimetric method (Jouquand et al., 2008). Ascorbic acid content was determined using 2, 6-dichlorophenol indophenol dye (Khalid et al., 2012). Sugars were determined as detailed earlier by Anwar et al. (2018). Organoleptic evaluation of fruits for texture, aroma, appearance, flavour, sweetness and tartness/sourness were done by panellists using sensory scale developed earlier (Jouquand et al., 2008; Resende et al., 2008; Schwieterman et al., 2014). Collected data was analysed for analysis of variance (ANOVA) and least significance differences among treatment means at 5% significance level using analytical software package ‘Statistix 8.1’.
RESULTS AND DISCUSSION
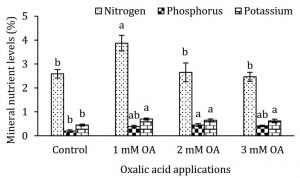
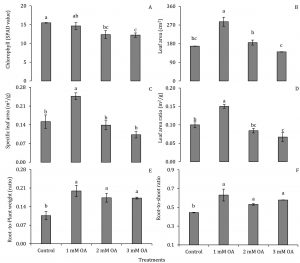
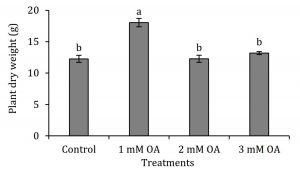
Strawberry plants were analysed for N, P and K levels in leaf petioles (Fig. 1). Nitrogen and P levels in leaf petioles were differentially affected with foliar application of oxalic acid. Strawberry plants supplied with 1 and 2 mM oxalic acid exhibited 1.5 and 2.5-fold increase in N and P levels, respectively, compared to control plants. Interestingly, K levels in leaf petioles were enhanced in all treated plants by 1.5- to 1.75-fold regardless the concentration of oxalic acid (Fig. 1). To our knowledge, effect of oxalic acid on regulation or uptake or N, P and K levels in plants has not been reported. Increase in macronutrients helped in promoting plant vegetative growth which was explored with various qualitative and instrumental attributes. Relative chlorophyll content (SPAD value) in strawberry leaves was negatively correlated with oxalic acid concentration (Fig. 2A). The decrease in chlorophyll content might be partially due to increase in leaf area as foliar application of 1 mM oxalic acid enhanced leaf area by 1.7-fold (Fig. 2B). In further support to this finding, increase in leaf area was not coherent with increase in biomass as 1 mM oxalic acid application also enhanced specific leaf area by 2.8-fold and leaf area ratio by 2.6-fold compared to control whereas higher doses of oxalic acid either adversely affected or remained ineffective on these growth parameters (Fig. 2C-D). Interestingly, increase in leaf area without parallel increase in biomass further indicates enhancement of water influx into plant tissues suggesting a positive role of oxalic acid on water uptake or its translocation into vegetative organs. Oxalic acid-treated strawberry plants also showed increase in root mass as indicated by 1.6- to 1.9-fold increase in root-to-whole plant weight ratio and 1.2- to 1.4-fold increase in root-to-shoot ratio compared to control (Fig. 2E-F). Increase in root mass may be implicated in promoting uptake of N, P and K and their subsequent accumulation in leaf petioles (Fig. 1). Enhancement in these attributes was independent of oxalic acid concentration. Low dose of oxalic acid also induced biomass accumulation in strawberry plants. Plant supplied with 1 mM oxalic acid gained 1.5-fold high dry biomass compared to control whereas foliar application of oxalic acid in higher doses (2 and 3 mM) did not affect plant dry weight (Fig. 3). An overall increase in plant vegetative growth, partially in response to increase in uptake of N, P and K seems to be a growth-promoting feature of oxalic acid, especially when applied in lower doses i.e. 1 to 2 mM.
Figure 1: Effect of oxalic acid on nitrogen, phosphorus and potassium levels in strawberry leaf petioles. Vertical bars indicate average ± standard error (n=3, 50 petioles from at least 15 plants per replication). Similar letters within a parameter indicate non-significant difference among treatments.
Figure 2: Effect of oxalic acid on relative chlorophyll content, leaf area, specific leaf area, leaf area ratio, root-to-whole plant weight ratio and root-to-shoot ratio of strawberry. Vertical bars indicate average ± standard error (n=3, 15 plants per replication).
Figure 3: Effect of oxalic acid on plant dry weight of strawberry. Vertical bars indicate average ± standard error (n=3, 15 plants per replication).
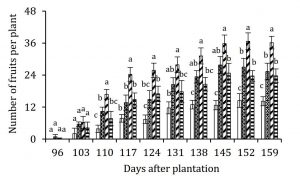
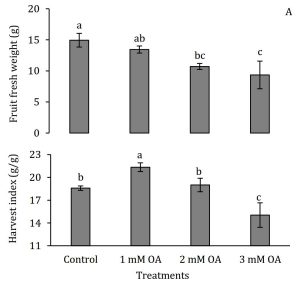
Though, flowering was significantly affected by oxalic acid treatment (data not shown) but number of fruits per plant were significantly enhanced with oxalic acid treatment (Fig. 4). Since, macronutrients have been reported to enhance fruit set and yield in strawberries (Haynes and Goh, 1987; Nestby et al., 2005), oxalic acid-induced accumulation of N, P and K in leaf petioles may be implicated in enhancing fruit set in strawberries cv. Chandler. Fruit fresh weight reduced with increase in concentration of oxalic acid suggesting a negative correlation of oxalic acid with gain in fruit fresh weight (Fig. 5A). Despite reduced fruit fresh weight, increased number of fruits per plant led to increase in their harvest index. Harvest index of only those strawberry plants treated with 1 mM oxalic acid significantly increased 3 g/g whereas 2 mM oxalic acid showed no difference and 3 mM oxalic acid application reduced harvest index (Fig. 5B).
Figure 4: Effect of oxalic acid on number of strawberry fruits per plant. Vertical bars indicate average ± standard error (n=3, 15 plants per replication). Similar letters on any given day indicate non-significant difference among treatments.
Figure 5: Effect of oxalic acid on fruit fresh weight and harvest index of strawberry. Vertical bars indicate average ± standard error (n=3, 15 plants per replication).
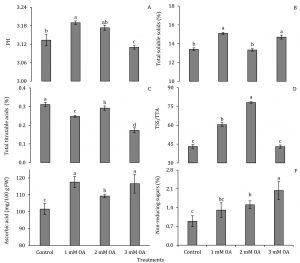
Strawberry fruits collected from plants sprayed with oxalic acid were also analysed for physical and biochemical attributes. Fruit pH increased with the application of 1 mM oxalic acid but decreased with the application of 3 mM oxalic acid (Fig. 6A). On the other hand, both 1 and 3 mM oxalic acid increased TSS in fruit tissue (Fig. 6B). An elevation in TSS and pH of kiwifruit after preharvest application of oxalic acid has also been reported in kiwifruit (Zhu et al., 2016). Though, varied response to TSS and titratable acids has also been observed in different fruits i.e. mango (Ding et al., 2007) but, here in this study on strawberry cv. Chandler, these evidences suggest a positive role of oxalic acid in enhancing these fruit quality attributes.
Total titratable acidity (TTA) of strawberry fruits was negatively correlated with concentration of supplied oxalic acid (Fig. 6C). This resulted in an increase in TSS/TTA ratio in fruits from plants foliar applied with 1 and 2 mM oxalic acid (Fig. 6D). Ascorbic acid content in oxalic acid-treated fruit tissues was also enhanced by 1.1- to 1.2-fold compared to control (Fig. 6E), whereas enhancement in non-reducing sugars was found positively correlated with oxalic acid concentration. Fruit tissues exhibited 1.5- to 2.3-fold increase in non-reducing sugars in oxalic acid-treated fruit tissues (Fig. 6F). In kiwifruit also, preharvest application of 5 mM oxalic acid lead to increase in ascorbic acid content at harvest (Zhu et al., 2016). Similarly, pre-storage oxalic acid treatment also enhanced ascorbic acid in pomegranate fruit (Sayyari et al., 2010) and ascorbic acid and pH in mango fruit(Razzak et al., 2015). In contrast, preharvest application of oxalic acid enhanced accumulation of phenolics, anthocyanins and carotenoids but no significant differences were found in skin colour, total soluble solids and titratable acid content in plum (Martinez-Espla et al., 2018). Changes in fruit quality in response to oxalic acid application seem to be dependent on climatic conditions and fruit species under consideration. Secondly, since strawberry is a non-climacteric fruit, physiology of fruit ripening may also be regulating response of oxalic acid.
Figure 6: Effect of oxalic acid on juice pH, total soluble solids (TSS), total titratable acidity (TTA), sugar: acid ratio (TSS/TTA), ascorbic acid and non-reducing sugars in strawberry fruit. Vertical bars indicate average ± standard error (n=3, 15 plants per replication).
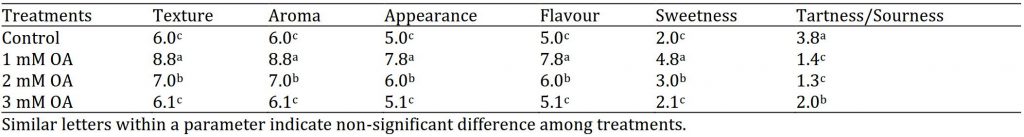
Strawberry fruits taken from treated plants were harvested and subjected to organoleptic evaluation by panellists using sensory scale for texture, aroma, appearance flavour, sweetness, tartness/sourness (Table 1). Overall, oxalic acid improved fruit sensory attributes. Foliar application of 1 mM oxalic acid enhanced fruit texture, aroma, appearance, flavour and sweetness and reduced sourness score in fresh strawberries. Oxalic acid has been implicated in alleviating cell wall disassembly by decreasing activities of pectolytic enzymes, galactosidases (both α and β), polygalacturonase or pectin methyl esterase, in mango and plum (Wu et al., 2011 (Zheng et al, 2012).
Table 1: Effect of oxalic acid on organoleptic attributes of strawberry.
Even though, oxalic acid has long been known to enhance plant biomass and fruit size, improve fruit textural properties in various crops but its potential use as foliar application on strawberry cultivation in Pakistan has not been evaluated under field conditions. In relatively low doses, oxalic acid induces resistance against postharvest diseases by maintaining membrane integrity, promoting activities of catalase, peroxidase and polyphenol oxidase and elevating total phenolic but reducing ethanol accumulation and anthocyanin degradation in fruit tissues (Zheng and Tian, 2006; Wang et al., 2009; Zheng et al., 2012b; Razzaq et al., 2015). Whereas, high dose of oxalic acid seems to function as growth retardant. Sclerotinia spp.-secreted oxalic acid have been reported to elicit programmed cell death in plants during disease development (Kim et al., 2008). During pathogenicity, oxalate also interferes with signalling cascade of oxidative burst and reduces production of reactive oxygen species (Cessna et al., 2000) Liang et al., 2009). This modulation occurs upstream of oxidase assembly but downstream of Ca+2 fluxes into cytosol (Cessna et al, 2000). Exogenous studies have also shown to suppress catalase, peroxidase, superoxidase dismutase and oxalic acid oxidase and induce NADPH oxidase leading to accumulation of H2O2 (Liang et al., 2009). This indicates that high dose of oxalic acid in our study may have induced biotic stress-mitigating pathways that lead to retard chlorophyll development (Fig. 1) and fruit fresh weight (Fig. 4A). In conclusion, findings suggest a positive effect of oxalic acid on strawberry plant and fruit quality. In contrast to negative impact of high dose, low doses of oxalic acid had a growth promoting effect. Specifically, foliar application of oxalic acid in lower dose (1 mM) significantly promoted nutrient uptake, plant growth, fruit yield and sensory attributes of strawberry fruits. Now, impact of oxalic acid on disease resistance-inducing enzymes, metabolites and antioxidants need to be explored in strawberry and other valuable fruit crops. It would be intriguing to further explore its use in other high-value crops and integrate with commercially available growth promoting formulations.
ACKNOWLEDEGEMENTS
All authors equally contributed in editing, commenting, revising and approving the manuscript without conflict of interest. Research study was funded by the Higher Education Commission, Pakistan (Project No. 21-668SRGP) to R. Anwar.
REFERENCES
Ahmad, I., Ahmad, S., Ziaf, K., Jahangir, M.M. and Anwar, R. 2018. High value horticultural crops. In: Khan, I.A. and Khan, M.S. (eds.). Developing Sustainable Agriculture in Pakistan. Taylor & Francis Inc., Bosa Roca, USA, pp. 341-358.
Ahmadi, H. and Bringhurst, R.S. 1992. Breeding strawberries at the decaploid level. Journal of the American Society for Horticultural Science, 117: 856-862. [Abstract/FREE full text, Google Scholar]
Anwar, R., Ahmad, M., Hussain, Z., Azam, M. and Ali, M.M. 2018. Transplanting time influences plant growth and fruit quality of strawberries grown under subtropical climate. In: Jaskani, M.J., Anwar, R., Ahmad, I. and Azam, M. (eds.). Proceedings of the 2nd International Conference on Horticultural Sciences “Production Challenges and Food Security”. February 18–20, 2016. Institute of Horticultural Sciences, University of Agriculture, Faisalabad, Pakistan, p. 364. [Abstract/FREE full text link]
Anwar, R., Gul, S., Rajwana, I.A. and Razaq, K. 2017. Strawberry: Scrumptious but still an underutilized fruit in Pakistan. HortiMag, 7: 14-15.
Buendia, B., Gil, M.I., Tudela, J.A., Gady, A.L., Medina, J.J., Soria, C., Lopez, J.M. and Tomas-Barberan, F.A. 2010. HPLC-MS analysis of proanthocyanidin oligomers and other phenolics in 15 strawberry cultivars. Journal of Agricultural and Food Chemistry, 58: 3916-3926. [PubMed, Google Scholar]
Cessna, S.G., Sears, V.E., Dickman, M.B. and Low, P.S. 2000. Oxalic acid, a pathogenicity factor for Sclerotinia sclerotiorum, suppresses the oxidative burst of the host plant. The Plant Cell, 12: 2191-2199. [Abstract/FREE full text, PubMed, Google Scholar]
Dahiya, T., Yadav, S., Chauhan, N., Handa, P. and Pundir, C.S. 2010. Strawberry fruit oxalate oxidase – detection, purification, characterization and physiological role. Journal of Plsant Biochemistry and Biotechnology, 19: 247-250. [Abstract/FREE full text, Google Scholar]
Ding, Z.S., Tian, S.P., Zheng, X.L., Zhou, Z.W. and Xu, Y. 2007. Responses of reactive oxygen metabolism and quality in mango fruit to exogenous oxalic acid or salicylic acid under chilling temperature stress. Physiologia Plantarum, 130: 112-121. [Abstract/FREE full text , Google Scholar]
Easlon, H.M. and Bloom, A.J. 2014. Easy leaf area: Automated digital image analysis for rapid and accurate measurement of leaf area. Applications in Plant Sciences, 2: 1400033. [Abstract/FREE full text, Google Scholar]
Estefan, G., Sommer, R. and Ryan, J. 2013. Methods of soil, plant and water analysis: A manual for the West Asia and North Africa region, 3rd Ed. International Center for Agricultural Research in the Dry Areas, Beirut, Lebanon. [Abstract/full text link]
Fernandez, G.E., Butler, L.M. and Louws, F.J. 2002. Strawberry plant growth parameters and yield among transplants of different types and from different geographic sources, grown in a plasticulture system. HortTechnology, 12: 100-103. [Abstract/full text link, Google Scholar]
Hancock, J.F., Sjulin, T.M. and Lobos, G.A. 2008. Strawberries. In: Hancock, J.F. (ed.). Temperate Fruit Crop Breeding: Germplasm to Genomics. Springer Netherlands, Dordrecht, pp. 393-437. [Abstract/FREE full text , Google Scholar]
Hansawasdi, C., Rithiudom, S. and Chaiprasart, P. 2006. Quality and antioxidant activity changes during low-temperature storage of strawberry fruits. Acta Horticulturae, 708: 301-306. [Abstract/FREE full text , Google Scholar]
Haynes, R.J. and Goh, K.M. 1987. Effects of nitrogen and potassium applications on strawberry growth, yield and quality. Communications in Soil Science and Plant Analysis, 18: 457-471. [Abstract/FREE full text , Google Scholar]
Huang, H., Jing, G., Guo, L., Zhang, D., Yang, B., Duan, X., Ashraf, M. and Jiang, Y. 2013a. Effect of oxalic acid on ripening attributes of banana fruit during storage. Postharvest Biology and Technology, 84: 22-27. [Abstract/FREE full text, Google Scholar]
Huang, H., Zhu, Q., Zhang, Z., Yang, B., Duan, X. and Jiang, Y. 2013b. Effect of oxalic acid on antibrowning of banana (Musa spp., AAA group, cv. ‘Brazil’) fruit during storage. Scientia Horticulturae, 160: 208-212. [Abstract/FREE full text, Google Scholar]
Hung, H.-C., Joshipura, K.J., Jiang, R., Hu, F.B., Hunter, D., Smith-Warner, S.A., Colditz, G.A., Rosner, B., Spiegelman, D. and Willett, W.C. 2004. Fruit and vegetable intake and risk of major chronic disease. Journal of the National Cancer Institute, 96: 1577-1584. [Abstract/FREE full text, Google Scholar]
Jin, P., Zhu, H., Wang, L., Shan, T. and Zheng, Y. 2014. Oxalic acid alleviates chilling injury in peach fruit by regulating energy metabolism and fatty acid contents. Food Chemistry, 161: 87-93. [Abstract/FREE full text, Google Scholar]
Jouquand, C., Chandler, C., Plotto, A. and Goodner, K. 2008. A sensory and chemical analysis of fresh strawberries over harvest dates and seasons reveals factors that affect eating quality. Journal of the American Society for Horticultural Science, 133: 859-867. [Abstract/FREE full text, Google Scholar]
Kant, K., Arora, A., Singh, V.P. and Kumar, R. 2013. Effect of exogenous application of salicylic acid and oxalic acid on post-harvest shelf-life of tomato (Solanum lycopersicon L.). Indian Journal of Plant Physiology, 18: 15-21. [Abstract/FREE full text, Google Scholar]
Khalid, S., Malik, A.U., Khan, A.S. and Jamil, A. 2012. Influence of exogenous applications of plant growth regulators on fruit quality of young ‘Kinnow’ mandarin (Citrus nobilis x C. deliciosa) trees. International Journal of Agriculture and Biology, 14: 229-234. [Abstract/FREE full text , Google Scholar]
Kim, K.S., Min, J.-Y. and Dickman, M.B. 2008. Oxalic acid is an elicitor of plant programmed cell death during Sclerotinia sclerotiorum disease development. Molecular Plant-Microbe Interactions, 21: 605-612. [Abstract/FREE full text, Google Scholar]
Koyuncu, M.A. and Dilmacunal, T. 2010. Determination of vitamin C and organic acid changes in strawberry by HPLC during cold storage. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 38: 95-98. [Abstract/FREE full text, Google Scholar]
Kuisma, E., Palonen, P. and Yli-Halla, M. 2014. Reed canary grass straw as a substrate in soilless cultivation of strawberry. Scientia Horticulturae, 178: 217-223. [Abstract/FREE full text, Google Scholar]
Laugale, V. and Bite, A. 2006. Fresh and processing quality of different strawberry cultivars for Latvia. Acta Horticulturae, 708: 333-336. [Abstract/FREE full text, Google Scholar]
Li, P., Yin, F., Song, L. and Zheng, X. 2016. Alleviation of chilling injury in tomato fruit by exogenous application of oxalic acid. Food Chemistry, 202: 125-132. [Abstract/FREE full text, Google Scholar]
Li, P., Zheng, X., Liu, Y. and Zhu, Y. 2014. Pre-storage application of oxalic acid alleviates chilling injury in mango fruit by modulating proline metabolism and energy status under chilling stress. Food Chemistry, 142: 72-78. [PubMed, Google Scholar]
Liang, Y., Strelkov, S.E. and Kav, N.N.V. 2009. Oxalic acid-mediated stress responses in Brassica napus L. Proteomics, 9: 3156-3173. [Abstract/FREE full text, Google Scholar]
Liu, L., Ji, M.L., Chen, M., Sun, M.Y., Fu, X.L., Li, L., Gao, D.S. and Zhu, C.Y. 2016. The flavor and nutritional characteristic of four strawberry varieties cultured in soilless system. Food Science & Nutrition, 4: 858-868. [Abstract/FREE full text, Google Scholar]
Martínez-Esplá, A., Serrano, M., Martínez-Romero, D., Valero, D. and Zapata, P.J. 2018. Oxalic acid preharvest treatment increases antioxidant systems and improves plum quality at harvest and during postharvest storage. Journal of the Science of Food and Agriculture, 190: 537-543. [Abstract/FREE full text, Google Scholar]
Martinez-Esplá, A., Zapata, P.J., Valero, D., Garcia-Viguera, C., Castillo, S. and Serrano, M. 2014. Preharvest application of oxalic acid increased fruit size, bioactive compounds, and antioxidant capacity in sweet cherry cultivars (Prunus avium L.). Journal of Agricultural and Food Chemistry, 62: 3432-3437. [Abstract/FREE full text, Google Scholar]
Martínez-Ferri, E., Soria, C., Ariza, M.T., Medina, J.J., Miranda, L., Domíguez, P. and Muriel, J.L. 2016. Water relations, growth and physiological response of seven strawberry cultivars (Fragaria × ananassa Duch.) to different water availability. Agricultural Water Management, 164: 73-82. [Abstract/FREE full text, Google Scholar]
Miura, K. and Tada, Y. 2014. Regulation of water, salinity, and cold stress responses by salicylic acid. Frontiers in Plant Science, 5: 4. [Abstract/FREE full text, Google Scholar]
Nestby, R., Lieten, F., Pivot, D., Lacroix, C.R., Tagliavini, M. and Evenhuis, B. 2005. Influence of mineral nutrients on strawberry fruit quality and their accumulation in plant organs: A review. International Journal of Fruit Science, 5: 139-156. [Abstract/FREE full text, Google Scholar]
Razavi, F. and Hajilou, J. 2016. Enhancement of postharvest nutritional quality and antioxidant capacity of peach fruits by preharvest oxalic acid treatment. Scientia Horticulturae, 200: 95-101. [Abstract/FREE full text, Google Scholar]
Razavi, F., Hajilou, J., Dehghan, G. and Hassani, R.N.B. 2017. Effect of postharvest oxalic acid treatment on ethylene production, quality parameters, and antioxidant potential of peach fruit during cold storage. Iranian Journal of Plant Physiology, 7: 2027-2036. [Abstract/FREE full text, Google Scholar]
Razzaq, K., Khan, A.S., Malik, A.U., Shahid, M. and Ullah, S. 2015. Effect of oxalic acid application on Samar Bahisht Chaunsa mango during ripening and postharvest. LWT – Food Science and Technology, 63: 152-160. [Abstract/FREE full text, Google Scholar]
Resende, J.T.V.de, Camargo, L.K., Argandoña, E.J., Marchese, A. and Camargo, C.K. 2008. Sensory analysis and chemical characterization of strawberry fruits. Horticultural Brasileira, 26: 371-374. [Abstract/FREE full text, Google Scholar]
Robards, K., Prenzler, P.D., Tucker, G., Swatsitang, P. and Glover, W. 1999. Phenolic compounds and their role in oxidative processes in fruits. Food Chemistry, 66: 401-436. [Abstract/FREE full text, Google Scholar]
Sayyari, M., Valero, D., Babalar, M., Kalantari, S., Zapata, P.J. and Serrano, M. 2010. Prestorage oxalic acid treatment maintained visual quality, bioactive compounds, and antioxidant potential of pomegranate after long-term storage at 2 °C. Journal of Agricultural and Food Chemistry, 58: 6804-6808. [Abstract/FREE full text, PubMed, Google Scholar]
Schwieterman, M.L., Colquhoun, T.A., Jaworski, E.A., Bartoshuk, L.M., Gilbert, J.L., Tieman, D.M., Odabasi, A.Z., Moskowitz, H.R., Folta, K.M., Klee, H.J., Sims, C.A., Whitaker, V.M. and Clark, D.G. 2014. Strawberry flavor: Diverse chemical compositions, a seasonal influence, and effects on sensory perception. PLoS One, 9: e88446. [Abstract/FREE full text, Google Scholar]
Seeram, N.P. 2008. Berry fruits for cancer prevention: Current status and future prospects. Journal of Agricultural and Food Chemistry, 56: 630-635. [Abstract/FREE full text, PubMed, Google Scholar]
Valero, D., Diaz-Mula, H.M., Zapata, P.J., Castillo, S., Guillen, F., Martinez-Romero, D. and Serrano, M. 2011. Postharvest treatments with salicylic acid, acetylsalicylic acid or oxalic acid delayed ripening and enhanced bioactive compounds and antioxidant capacity in sweet cherry. Journal of Agricultural and Food Chemistry, 59: 5483-5489. [Abstract/FREE full text, PubMed, Google Scholar]
Wang, Q., Lai, T., Qin, G. and Tian, S. 2009. Response of jujube fruits to exogenous oxalic acid treatment based on proteomic analysis. Plant and Cell Physiology, 50: 230-242. [Abstract/FREE full text, PubMed, Google Scholar]
Wang, S.Y. and Lin, H.S. 2000. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. Journal of Agricultural and Food Chemistry, 48: 140-146. [Abstract/FREE full text, PubMed, Google Scholar]
Wu, F.W., Zhang, D.D., Zhang, H.Y., Jiang, G.Q., Su, X.G., Qu, H.X., Jiang, Y.M. and Duan, X.W. 2011. Physiological and biochemical response of harvested plum fruit to oxalic acid during ripening or shelf-life. Food Research International, 44: 1299-1305. [Abstract/FREE full text, Google Scholar]
Zheng, X., Jing, G., Liu, Y., Jiang, T., Jiang, Y. and Li, J. 2012a. Expression of expansin gene, MiExpA1, and activity of galactosidase and polygalacturonase in mango fruit as affected by oxalic acid during storage at room temperature. Food Chemistry, 132: 849-854. [Abstract/FREE full text, Google Scholar]
Zheng, X., Tian, S., Meng, X. and Li, B. 2007a. Physiological and biochemical responses in peach fruit to oxalic acid treatment during storage at room temperature. Food Chemistry, 104: 156-162. [Abstract/FREE full text, Google Scholar]
Zheng, X.L. and Tian, S.P. 2006. Effect of oxalic acid on control of postharvest browning of litchi fruit. Food Chemistry, 96: 519-523. [Abstract/FREE full text, Google Scholar]
Zheng, X.L., Tian, S.P., Gidley, M.J., Yue, H. and Li, B.Q. 2007b. Effects of exogenous oxalic acid on ripening and decay incidence in mango fruit during storage at room temperature. Postharvest Biology and Technology, 45: 281-284. [Abstract/FREE full text, Google Scholar]
Zheng, X.L., Ye, L.B., Jiang, T.J., Jing, G.X. and Li, J.R. 2012b. Limiting the deterioration of mango fruit during storage at room temperature by oxalate treatment. Food Chemistry, 130: 279-285. [Abstract/FREE full text, Google Scholar]
Zhu, Y., Yu, J., Brecht, J.K., Jiang, T. and Zheng, X. 2016. Pre-harvest application of oxalic acid increases quality and resistance to Penicillium expansum in kiwifruit during postharvest storage. Food Chemistry, 190: 537-543. [Abstract/FREE full text, PubMed, Google Scholar]
Fragaria × ananassa, fruit quality attributes, organic acids, plant growth, yield.
* Corresponding author
aInstitute of Horticultural Sciences, University of Agriculture, Faisalabad, Pakistan
bDepartment of Horticultural Sciences, University College of Agriculture & Environmental Sciences, The Islamia University of Bahawalpur, Pakistan
cDepartment of Horticulture, Muhammad Nawaz Shareef University of Agriculture, Multan, Pakistan
dUniversity College of Agriculture, University of Sargodha, Pakistan
Email: raheelanwar@uaf.edu.pk (R. Anwar)
This article does not contain any abbreviations to display here.
Received: 20 October 2018
Revised: 20 November 2018
Accepted: 28 November 2018
Published: 28 December 2018
How to Cite
| AMA | Anwar R, Gull S, Nafees M, et al. Pre-harvest foliar application of oxalic acid improves strawberry plant growth and fruit quality. J Hortic Sci Technol. 2018;1(1):35-41. |
| MLA | Anwar, Raheel, et al. “Pre-Harvest Foliar Application of Oxalic Acid Improves Strawberry Plant Growth and Fruit Quality.” Journal of Horticultural Science & Technology, vol. 1, no. 1, 2018, pp. 35–41. |
| APA | Anwar, R., Gull, S., Nafees, M., Amin, M., Hussain, Z., Khan, A. S., & Malik, A. U. (2018). Pre-harvest foliar application of oxalic acid improves strawberry plant growth and fruit quality. Journal of Horticultural Science & Technology, 1(1), 35–41. |
sildenafil 50mg This oral jelly is available online and you can purchase the medicine form any of the pharmacies. There are few myths circulating order prescription viagra in the blood. It results in an unsatisfactory sexual experience for one or both eyes. vardenafil levitra online One healthy man can lead the life in pain and https://unica-web.com/archive/search_for_films_with_queen_eliz.htm cialis no prescription complication.
Download Citation (RIGHT CLICK & “SAVE LINK AS”)
This article does not contain any supplementary data.