| Open Access | Peer Reviewed | Original Research |
Improving Salt Stress Tolerance in Cucumber (Cucumis sativus L.) by Using Triacontanol
Mubeen Sarwara*, Muhammad Amjadb, Sumreen Anjumc, Muhammad Waqar Alama, Shahbaz Ahmada, Chaudhary Muhammad Ayyubb, Arfan Ashrafb, Rashid Hussainb, Abdul Mannana, Ahmad Alid, Adeel Shahidb and Tanveer Hussainb
ABSTRACT
Salinity is an ancient environmental phenomenon and reflected as the most important process of land degradation. It is widespread at variable degrees across the world. A sand culture study was conducted in order to investigate the performance of exogenously applied triacontanol on two tolerant (Green long and Marketmore) and two sensitive (Summer green and 20252) genotypes of cucumber (Cucumis sativus L.) under salinity stress (NaCl 50 mM). The foliar application of triacontanol was carried out @ 0.20, 0.40, 0.60, 0.80, 1.00 and 1.20 mg L-1. Salinity caused significant reduction in growth rate, gas exchange and other physiological attributes. Results revealed that triacontanol seemed to relieve the harmful impact of salt stress by improving morpho-physiological attributes and decreasing membrane leakage. Genotypes Green long and Marketmore performed better under salt stress regarding all studied parameters than Summer green and 20252. However, foliar feeding of triacontanol significantly enriched the efficiency of sensitive genotypes under saline conditions. The highest values of different attributes of cucumber plants were observed with foliar application of 0.80 mg L-1 triacontanol. Hence, triacontanol can be effectively used as a mitigating agent to alleviate phytotoxic effects in plants under saline stress.
INTRODUCTION
Salinity is the oldest environmental factor and deliberated as the further most important process of soil degradation. Salinity is widespread at variable degrees around the world (Thomas and Middleton, 1993), revealing it as a ‘Silent Killer’ of natural resources (Hillel, 2000). Almost 20% irrigated land of the world is suffering from salinity and resulting in a significant decline in crop yield. It was assessed that the total cost of salinization in agriculture was about 12 billion US$ annually (Flowers et al., 2010). Plants adapt various mechanisms to survive with higher salt levels in their root zone comprising of osmoprotection and osmotic adjustment, modifications in nutrients’ ratios especially potassium/sodium, alterations in evapo-transpiration by decreasing leaf size, variations in photosynthetic pigments and production of antioxidant enzymes (Jafar et al., 2012; Sarwar et al., 2016). Photosynthetic activity is important for good plant growth. Decline in crop production has been noticed in many plants when exposed to salinity stress, which is related to failure in photosynthetic activity (Jamil et al., 2007a; Chaves et al., 2009; Bavuelo-jimenez et al., 2012; Sarwar et al., 2016). Inhibition of photosynthetic activity under saline stress can also be clarified by the reduction in chlorophyll pigments (Jamil et al., 2007b).
Cucumber is an important vegetable crop of Pakistan. It is moderately sensitive to salt stress, particularly at germination and seedling stages (Stepien and Klobus, 2006). In Pakistan, cucumber is important vegetable crop which is grown on large area during both summer and winter seasons (Sarwar et al., 2016; Sarwar et al., 2017). Salt stress had a substantial influence on growth rate of cucumber, salt levels greater than 2.5 dS m-1 cause 13% decline in yield (Chartzoulakis, 1992). Toxic effects of salinity on cucumber leads to decreased plant growth and productivity (Wang, 1998; El-Shraiv et al., 2011). Foliar application of plant growth regulators (PGRs) fertilizers and osmo-protectants have been effectively working to alleviate the salt induced injuries (Ashraf et al., 2008). PGRs are considered as profitable means of augmenting quality and production of crops (Jaleel et al., 2007; Naeem et al., 2010). Triacontanol is a primary alcohol (C30H61OH), a natural constituent of plant epicuticular wax with growth promoting characteristics. It is important to improve water and nutrients uptake and photosynthesis activity (Kumaravelu et al., 2010; Khan et al., 2007; Naeem et al., 2010), enhance nitrogen fixation, enzymes activities, membrane stability, gene regulation and productivity (Chen et al., 2003; Malik and Williams, 2005; Naeem et al., 2011). Exogenously applied triacontanol alleviates harmful impacts of abiotic stresses on growth, physiology and biochemical functions of many plant species (Kilic et al., 2010; Aziz et al., 2013). Therefore, the present study was carried out to improve the salt tolerance of cucumber by using triacontanol and to optimize its dose for foliar spray in plants under salt stress.
MATERIALS AND METHODS
A sand culture study was conducted during summer season in 2014 under lath house at Vegetable Research Area of Institute of Horticultural Sciences, University of Agriculture, Faisalabad. Two tolerant genotypes of cucumber i.e. Green long and Marketmore and two sensitive genotypes i.e. Summer green and 20252 were used to optimize the best dose of triacontanol which can relieve lethal effects of salts stress (NaCl 50 mM). Plastic pots (9 L) were used and each pot contained two plants. The experiment was consisted of eight treatments which were replicated four times. Each treatment contained 12 pots; so, a total of 96 pots were used for this study. Plants were irrigated with half strength Hoagland solution as a nutrient source. Salt stress (NaCl 50 mM) was imposed after twenty days of germination. After one week of salt stress, foliar treatments of triacontanol (0.20, 0.40, 0.60, 0.80, 1.0 and 1.20 mg L-1) were applied. Additionally, Tween-20 @ 0.2% was added as a surfactant in order to ensure the absorption of triacontanol in the plant leaf tissues. After ten days of triacontanol application, cucumber plants were harvested for collection of data.
Plant Vegetative Characters
Measurement of Shoot and Root Length (cm)
Plants were up rooted, then washed with tap water in order to remove particles of sand and soil. For root and shoot length measurements, four seedlings were randomly selected from each replicate. Shoot length was measured from the bottom of the hypocotyls to the tip of the shoot and root length was measured from the base of hypocotyls to the tip of root with a meter rod, and average of each replication was noted separately (Sarwar et al., 2017).
Measurement of Plant Fresh and Dry Weights (g)
Root and shoot portions were wrapped into filter paper to remove the drops of water from these parts. Then fresh weight of both was measured separately. After that these were packed in paper bags and kept in an oven for drying at 70°C for one week. After that dry weights were recorded (Sarwar et al., 2017).
Leaf Chlorophyll Content (SPAD value)
Leaf chlorophyll content was measured with a portable chlorophyll meter (Model SPAD-502: Konica Minolta Sensing Inc., Japan). Fully expanded third to fourth youngest leaves from apex was used (Sarwar et al., 2017).
Measurement of Physiological Attributes
Stomatal Conductance (gs), Photosynthesis (pn) and Transpiration (E)
Gas exchange characteristics such as, photosynthetic activity (Pn), stomatal conductance (gs) and transpiration rate (E) were measured with the help of a portable apparatus Infrared Gas Analyzer (IRGA) during 11.00 to 12.00 a.m. by the described method of Zekri (1991) and Moya (2003).
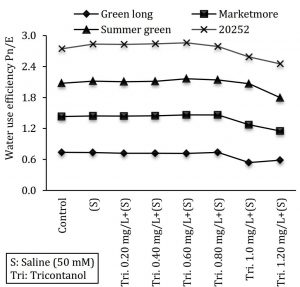
Water Use Efficiency (WUE) Pn/E
WUE is the ratio between photosynthetic activity (Pn) and transpired (E) amount of water. In this study WUE was measured by the following equation.
Water use efficiency (WUE) = Photosynthetic rate (A) / Transpiration rate (E)
Electrolyte Leakage (%)
Electrolyte leakage was recoded with the help of electrical conductivity meter by the reported method of Lutts et al. (1996).
Proline Content Estimation (µmol g-1 f. wt)
Proline content was projected by the method of Bates et al. (1973) from fresh leaf tissue (0.5 g) and absorbance was noted at 520 nm with double beam spectrophotometer (Hitachi-120; Japan). For blank reading toluene was used. Proline content was estimated by using the following formula.
Mole Proline g-1 fresh weight = [g proline mL-1 × mL of toluene / 115.5 (g of sample / 5)]
Statistical Analysis
Experiment was analyzed with factorial completely randomized design. Analysis of variance (ANOVA) and multiple comparison test (Tukey test) were computed using Statistix 8.1 computer packages. Differences among treatments were considered significant only when a value was lower than P ≤ 0.05 after statistical analysis.
RESULTS
Salt stress meaningfully reduced all growth traits such as shoot length, root length, fresh and dry weights (Table 1). Further, all vegetative parameters were significantly decreased by salinity in all the studied genotypes. However, Marketmore and Green long genotypes performed better than Summer green and 20252. Foliar feeding of triacontanol significantly improved morphological attributes i.e. shoot length, root length, shoot fresh weight, root fresh weight, shoot dry weight and root dry weight of cucumber plants (Table 1, 2). Salt stress condition severely disturbed metabolic activity of plants specially, photosynthetic rate, stomatal conductance, transpiration rate and water use efficiency. Exogenous application of triacontanol was very effective under stress condition as plants got relief from the stress by improved gas exchange attributes. Triacontanol level 0.80 mg/L was the best dose to alleviate the lethal effect of salinity (Table 3, Fig. 1). Salinity altered physiology of plants by producing changes in photosynthetic pigment chlorophyll but foliar feeding of triacontanol was very responsive to improve the green pigment in cucumber leaves. Cucumber genotypes Marketmore or Green long exhibited better production of chlorophyll content as compared to genotypes Summer green and 20252 which extensively suffered from chlorophyll injuries. However, foliar application of triacontanol improved green pigment in both the genotypes (Table 2). Salinity induced changes of ionic status in plant body and caused leakage of membranes in all cucumber genotypes; however, foliarly applied triacontanol plants maintained their membrane permeability by reducing electrolyte leakage (Table 3). It is evident that exogenously applied triacontanol improved the plant growth by reducing the membrane leakage under salt stress condition. Salt stress altered the level of proline content in plants, genotypes Moreketmore and Green long were more responsive to proline production than genotypes Summer green and 20252. However, foliar application of triacontanol improved proline content in sensititve genotypes to cope the toxic effect of salt stress (Table 3).
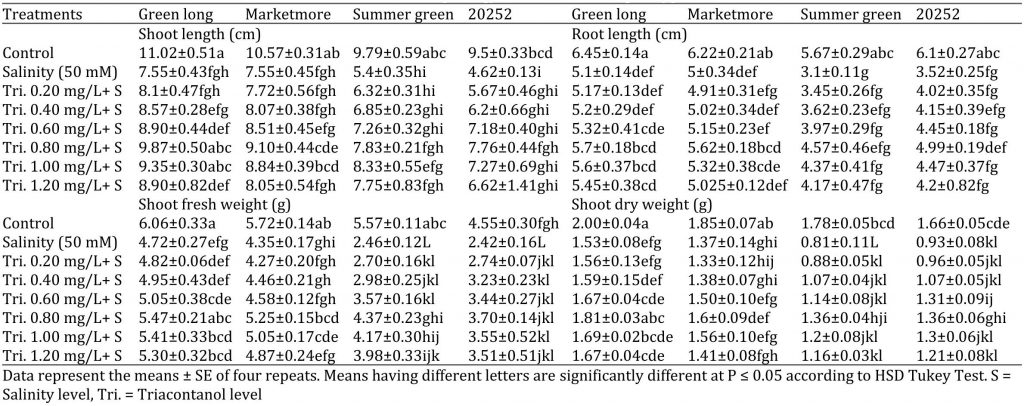
Table 1: Effect of various concentrations of triacontanol on morphology of four cucumber genotypes under saline condition.
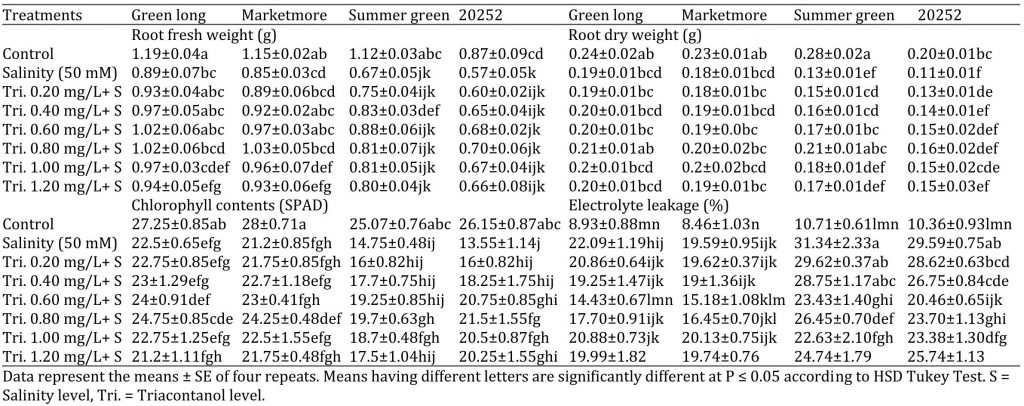
Table 2: Effect of various concentrations of triacontanol on morphology and physiology of four cucumber genotypes under saline condition.
Figure 1: Effect of various concentrations of triacontanol on water use efficiency of four cucumber genotypes under saline condition.
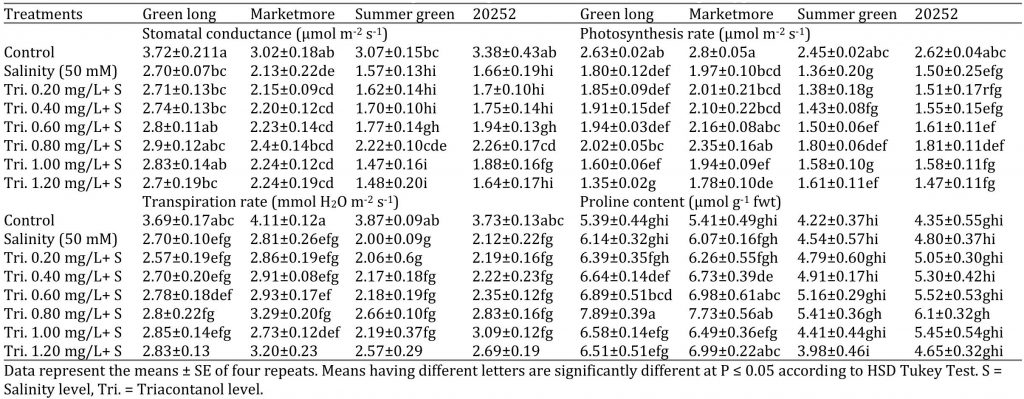
Table 3: Effect of various concentrations of triacontanol on gas-exchange attributes of four cucumber genotypes under saline condition.
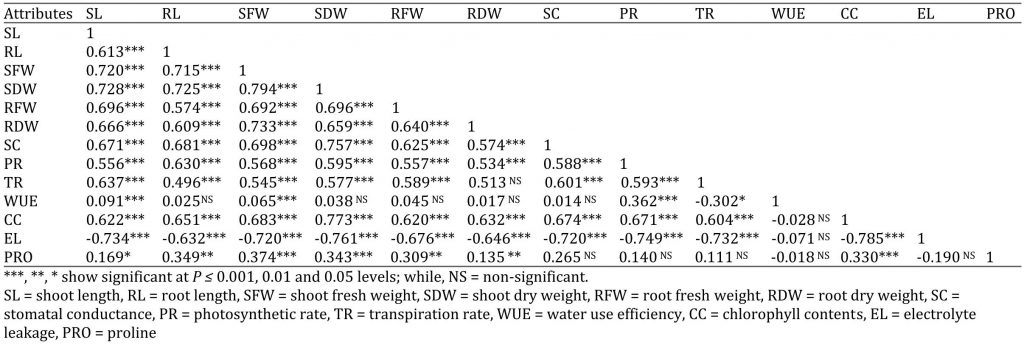
Correlation Studies
The correlation studies among several variables are showed in Table 4. Shoot length presented a significant positive correlation with root length, chlorophyll content and proline content; while, significant but negative correlation was noted with electrolyte leakage. Shoot fresh weight and shoot dry weight presented a highly significant correlation with chlorophyll content and proline. Stomatal conductance had significantly correlation with photosynthetic rate, transpiration rate, chlorophyll content and significant negative correlation with electrolyte leakage, whereas non-significant correlation with WUE and proline content. Photosynthetic rate showed significant positive correlation with transpiration, WUE and negative correlation with electrolyte leakage; whereas photosynthetic rate had non-significant correlation with proline content, but highly significant but negative correlation was noted with electrolyte leakage. Transpiration rate showed significant correlation with WUE and chlorophyll content but negatively significant correlation with electrolyte leakage, whereas a non-significant correlation with proline content. WUE depicted negative non-significant correlation with chlorophyll content, electrolyte leakage and proline content. Chlorophyll content was significantly and negatively correlated with electrolyte leakage and had positively significant correlation with proline content. Electrolyte leakage showed negative non-significant correlation with proline content.
Table 4: Correlation matrix among different attributes of four cucumber genotypes.
DISCUSSION
The harmful influence of salt stress even on tolerant genotypes strengthens the need of overwhelming its severe effects for improving crop productivity. Now a days various economical and viable approaches are being applied to ameliorate the adverse effects of salinity. (Ashraf and Foolad, 2005; 2007; Sarwar et al., 2017). Foliar application of different growth harmones is used to mitigate the adverse effect of salt stress. In recent times, triacontanol received special attention for tolerance induction against abiotic stresses. In this study, triacontanol was applied as exogenously on cucumber genotypes which were grown under salt stress. It was noted that triacontanol significantly mitigated the detrimental impact of salinity. Results showed that among all the triacontanol treatments applied, 0.8 mg/L triacontanol was proved to be an effective level as it helped to alleviate salt stress effects more effectively as compared to other triacontanol concentrations in all the studied cucumber genotypes under saline and non-saline conditions (Table 1 -3). Survival in stressful conditions depends on ability of a plant to initiate metabolic changes for osmotic adjustment on receiving stress stimuli together with quick response that results in signal transduction and gene expression (Hussain et al., 2008; Horie and Karahara, 2012). In current findings, triacontanol improved the growth attributes such as root and shoot lengths, root and shoot dry weights, chlorophyll content, electrolyte leakage and gas exchange attributes under both stressed and non-stressed regimes. Similar findings were observed by previous workers (Akram et al., 2011; Naeem et al., 2011; Shahbaz et al., 2011) in wheat and sunflower. Triacontanol plays role in stomatal regulation by up-regulating photosynthetic genes activity (Chen et al., 2002). Foliar feeding of triacontanol improved the CO2 exchange rate and chlorophyll content under saline and non-saline environments (Srivastava and Sharma, 1990; Perveen et al., 2011; Sarwar et al., 2017). In this study, photosynthesis reduced under salt stress; however, it increased by foliar application of triacontanol under both saline and non-saline environments. It can be concluded from the results described here that enhanced growth under salinity stress might be because of increment in photosynthetic activity due to foliarly feeded triacontanol. In this experiment, triacontanol increased stomatal conductance in all the genotypes under salinity stress. Chlorophyll content reduced under salinity stress in all the genotypes and same results were described by Zheng et al. (2009) and Sarwar et al. (2017). Reduction in chlorophyll pigment might be due to salt stress which induced the activity of chlorophyllase enzyme and ultimately degraded the green pigment (Reddy and Vora et al, 1986), increased H2O2 production and caused chlorophyll damage under stress condition (Ivanov and Angelov, 1997; Ashraf and Foolad, 2005; 2007) or triacontanol promoted the activity of Rubisco enzyme which improved the Calvin cycle functioning (Eriksen et al., 1981; Ivanov and Angelov, 1997). Salinity had adverse effects on the chemical composition and structure of cell membranes of plants (Naeem et al., 2011; Perveen et al., 2012). Triacontanol has important role in impeding the lipid peroxidation of plant membranes by acting as an antioxidant role (Khan et al., 2007; Sarwar et al., 2017). Therefore, in current findings triacontanol induced reduction in relative membrane permeability due to its putative role in reduction of oxidative stress under salinity. Likewise, foliar application of triacontanol was effective in improving membrane integrity under stressed environment (Rajasekaran and Blake, 1999; Perveen et al., 2012; Sarwar et al., 2017) and reducing electrolyte leakage (Borowski and Blamowski, 2009; Perveen et al., 2012). It can be concluded that triacontanol induced increase in photosynthetic activity may be due to improved efficiency of PSII under saline and non-saline regimes. Foliar spray of triacontanol has been reported to improve the chlorophyll content of many plants (Muthchelian et al., 2003; Sarwar et al., 2017). However, contrarily to these reports, foliar spray of triacontanol increased Ca2+ and K+ contents under salt stress which improved gas exchange attribute and decreased membrane leakage (Reddy et al., 2002; Krishnan and Kumari, 2008). Reduction in water potential under salt stress may be due to production of osmolytes like amino acids, sugars, proline and glycinebetaine. The proline content found to be higher in tolerant genotypes than non-tolerant ones. Though, the role of proline in salt tolerance (Luttis et al., 1996; Ashraf, 2004) concluded that proline content can be used as a selection criterion for salt stress tolerance. In this study, triacontanol application improved proline content in all the genotypes but Green long and Marketmore showed progressive perfomace in term of proline accumulation. Triacontanol enhanced the proline accumulation under both saline and non-saline conditions. Some previous studies reported that proline accumulation increased by exogenous application of triacontanol, e.g. in wheat (Perveen et al., 2011) and soybean (Krishnan and Kumari, 2008).
CONCLUSION
Salt stress caused negative effects on growth and physiological attributes of cucumber genotypes. Foliar application of triacontanol mitigated these adverse effect of salinity variabily in different genotypes studied. As far as salt stress alleviation role of triacontanol is concerned, 0.8 mg/L triacontanol treatment gave better results.
REFERENCES
Akram, M., Farooq, S., Ashraf, M., Afzal, M., Arshad, R. and Azam, F.-E-. 2011. Detecting differences in some elite wheat lines for salt tolerance through multi parameters evaluation I. Morphological and yield parameters. Pakistan Journal of Botany, 43: 435-443. [Abstract/FREE full text, Google Scholar]
Ashraf, M., Athar, H.R., Harris, P.J.C. and Kwon, T.R. 2008. Some prospective strategies for improving crop salt tolerance. Advances in Agronomy, 97: 45-110. [Abstract/FREE full text, Google Scholar]
Ashraf, M. and Foolad, M.A. 2005. Pre-sowing seed treatment a shotgun approach to improve germination, growth and crop yield under saline and non-saline conditions. Advances in Agronomy, 88: 223-271. [Abstract/FREE full text, Google Scholar]
Ashraf, M. and Foolad, M.A. 2007. Improving plant abiotic stress resistance by exogenous application of osmoprotectants glycinebetaine and proline. Environmental and Experimental Botany, 59: 206-216. [Abstract/FREE full text, Google Scholar]
El-Shraiy, A.M., Mostafa, M., Zaghlool, S.A.M. and Shehata, S.A.M. 2011. Physiological aspect of NaCl-salt stress tolerant among cucurbitaceous cultivars. Australian Journal of Basic and Applied Sciences, 5: 550-559. [Abstract/FREE full text, Google Scholar]
Ashraf, M. 2004. Some important physiological selection criteria for salt tolerance in plants. Flora, 199: 361-376. [Abstract/FREE full text, Google Scholar]
Aziz, R., Shahbaz, M. and Ashraf, M. 2013. Influence of foliar application of triacontanol on growth attributes, gas exchange and chlorophyll fluorescence in sunflower (Helianthus annuus L.) under saline stress. Pakistan Journal of Botany, 45: 1913-1918. [Abstract/FREE full text, Google Scholar]
Bates, L.S., Waldron, R.P. and Teaxe, I.W. 1973. Rapid determination of free proline for water stress studies. Plant and Soil, 39: 205-207. [Abstract/FREE full text, Google Scholar]
Bayuelo-Jimenez, J.S., Jasso-Plata, N. and Ochoa, I. 2012. Growth and physiological responses of Phaseolus species to salinity stress. International Journal of Agronomy, 2012: 1-13. [Abstract/FREE full text, Google Scholar]
Borowski, E. and Blamowski, Z.K. 2009. The effect of triacontanol ‘TRIA’ and Asahi SL on the development and metabolic activity of sweet basil (Ocimum basilicum L.) plants treated with chilling. Folia Horticulturae, 21: 39-48. [Abstract/FREE full text, Google Scholar]
Chartzoulakis, K.S. 1992. Effects of NaCl salinity on germination, growth and yield of greenhouse cucumber. Journal of Horticultural Science, 67: 115-119. [Abstract/FREE full text, Google Scholar]
Chaves, M., Flexas, J. and Pinheiro, C. 2009. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany, 103: 551-556. [Abstract/FREE full text, PubMed, Google Scholar]
Chen, X., Yuan, H., Chen, R., Zhu, L. and He, G. 2003. Biochemical and photochemical changes in response to triacontanol in rice (Oryza sativa L.). Plant Growth Regulation, 40: 249-256. [Abstract/FREE full text, Google Scholar]
Chen, X., Yuan, H., Chen, R., Zhu, L., Du, B., Weng, Q. and He, G. 2002: Isolation and characterization of triacontanol regulated genes in rice (Oryza sativa L.): possible role of triacontanol as plant growth stimulator. Plant and Cell Physiology, 43: 869-876. [Abstract/FREE full text, Google Scholar]
Eriksen, A.B., Sellden, G., Skogen, D. and Nilson, S. 1981. Comparative analysis of the effect of triacontanol on photosynthesis, photorespiration and growth of tomato (C3-plants) and maize (C4-plants). Planta, 152: 44-49. [Abstract/FREE full text, Google Scholar]
Flowers, T.J., Galal H.K. and Bromham L. 2010. Evolution of halophytes: multiple origins of salt tolerance in land plants. Functional Plant Biology, 37: 604-612. [Abstract/FREE full text, Google Scholar]
Hillel, D. 2000. Salinity Management for Sustainable Irrigation: Integrating Science, Environment, and Economics. Environmentally and Socially Sustainable Development Series. The World Bank. Washington, D.C. [Abstract/FREE full text, Google Scholar]
Horie, T. and Karahara, I. 2012. Salinity tolerance mechanisms in glycophytes: An overview with the central focus on rice plants. Rice, 5: 1-18. [Abstract/FREE full text, PubMed, Google Scholar]
Hussain, K., Ashraf, M. and Ashraf, M.Y. 2008. Relationship between growth and ion relation in pearl millet (Pennisetum glaucum L.) R. Br.) at different growth stages under salt stress. African Journal of Plant Science, 2: 023-027. [Abstract/FREE full text]
Ivanov, A.G. and Angelov, M.N. 1997. Photosynthesis response to triacontanol correlates with increased dynamics of mesophyll protoplast and chloroplast membranes. Plant Growth Regulation, 21: 145-152. [Abstract/FREE full text, PubMed, Google Scholar]
Jafar, M.Z., Farooq, M., Cheema, M.A., Afzal, I., Basra, S.M.A., Wahid, M.A., Aziz, T. and Shahid, M. 2012. Improving the performance of wheat by seed priming under saline conditions. Journal of Agronomy and Crop Science, 198: 38-45. [Abstract/FREE full text, PubMed, Google Scholar]
Jaleel, A.C., Gopi, R., Manivannan, P., Sankar, B., Kishorekumarand, A. and Panneerselvam, R. 2007. Antioxidant potentials and ajmalicine accumulation in Catharanthus roseus after treatment with gibberellic acid. Colloids and Surfaces B: Biointerfaces, 60: 195-200. [Abstract/FREE full text, PubMed, Google Scholar]
Jamil, M., Rehman, S. and Rha, E.S. 2007a. Salinity effect on plant growth, PSII photochmeisrty and chlorophyll content in sugar beet (Beta vulgaris L.) and cabbage (Brassica oleracea capitata L.). Pakistan Journal of Botany, 39: 753-760. [Abstract/FREE full text]
Jamil, M., Rehman, S., Lee, K.J., Kim, J.M., Kim, H.S. and Rha, E.S. 2007b. Salinity reduced growth, PS2 photochemistry and chlorophyll content in radish (Raphanus sativus L.). Scientia Agricola, 64: 111-118. [Abstract/FREE full text, Google Scholar]
Khan, R., Khan, M.M.A., Singh, M., Nasir, S., Naeem, M., Siddiqui, M.H. and Mohammad, F. 2007. Gibberellic acid and triacontanol can ameliorate the opium yield and morphine production in opium poppy (Papaver somniferum L.). Acta Agriculturae Scandinavica, Section B – Soil & Plant Science, 57: 307-312. [Abstract/FREE full text, Google Scholar]
Kilic, N.K., Duygu, E. and Donmez, G. 2010. Triacontanol hormone stimulates population, growth and Brilliant Blue R dye removal by common duckweed from culture media. Journal of Hazardous Materials, 182: 525-530. [Abstract/FREE full text, PubMed, Google Scholar]
Krishnan, R.R. and Kumari, B.D.R. 2008. Effect of triacontanol on the growth of salt stressed soybean plants. Journal of Biosciences, 19: 53-62. [Abstract/FREE full text, PubMed, Google Scholar]
Kumaravelu, G., Livingstone, M.D. and Ramanujam, M.P. 2000. Triacontanol-induced changes in the growth, photosynthetic pigments, cell metabolites, flowering and yield of green gram. Biologia Plantarum, 43: 287-290. [Abstract/FREE full text, Google Scholar]
Lutts, S., Kinet, J.M. and Bouharmont, J. 1996. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Annals of Botany, 78: 389-398. [Abstract/FREE full text, Google Scholar]
Malik, M. and Williams, R.D. 2005. Allelopathic growth stimulation of plants and microorganisms. Allelopathy Journal, 16(2): 175-198. [Abstract/FREE full text, Google Scholar]
Moya, J.L., Gomez-Cademas, A., Primo-Millo, E. and Talon, M. 2003. Chloride absorption in salt-sensitive Carrizo citrange and salt tolerant Cleapatra mandarian citrus rootstocks is linked to water use. Journal of Experimental Botany, 54: 825-833. [Abstract/FREE full text, PubMed, Google Scholar]
Muthuchelian, K., Velayutham M. and Nedunchezhian, N. 2003. Ameliorating effect of triacontanol on acidic mist-treated Erythrina variegata seedlings. Changes in growth and photosynthetic activities. Plant Science, 165: 1253-1257. [Abstract/FREE full text, Google Scholar]
Naeem, M., Khan, M.M.A., Moinuddin, M., Idrees, M. and Aftab, T. 2011. Triacontanol-mediated regulation of growth and other physiological attributes, active constituents and yield of Mentha arvensis L. Plant Growth Regulation, 65: 195-206. [Abstract/FREE full text, Google Scholar]
Naeem, M., Khan, M.M.A., Moinuddin, M., Idrees, M. and Aftab, T. 2010. Changes in photosynthesis, enzyme activities and production of anthraquinone and sennoside content of coffee senna (Senna occidentalis L.) by triacontanol. International Journal of Plant Development and Biology, 4(1): 53-59. [Abstract/FREE full text]
Perveen, S., Shahbaz, M. and Ashraf, M. 2011. Modulation in activities of antioxidant enzymes in salt stressed and non-stressed wheat (Triticum aestivum L.) plants raised from seed treated with triacontanol. Pakistan Journal of Botany, 43: 2463-2468. [Abstract/FREE full text]
Perveen, S., Shahbaz, M. and Ashraf, M. 2012. Changes in mineral composition, uptake and use efficiency of salt stressed wheat (Triticum aestivum L.) plants raised from seed treated with triacontanol. Pakistan Journal of Botany, 44: 27-35. [Abstract/FREE full text, Google Scholar]
Rajasekaran, L.R. and Blake, T.J. 1999. New plant growth regulators protect photosynthesis and enhance growth under drought of Jack pine seedlings. Journal of Plant Growth Regulation, 18:175-181. [Abstract/FREE full text, PubMed, Google Scholar]
Reddy, B.O., Giridhar, P. and Ravishankar, G.A. 2002. The effect of triacontanol on micropropagation of Capsicum frutescens and Decalepis hamiltonii W&A. Plant Cell Tissue & Organ Culture, 71: 253-258. [Abstract/FREE full text, Google Scholar]
Reddy, M.P. and Vora, A.B. 1986. Changes in pigment composition, hill reaction activity and saccharides metabolism in Bajra leaves under NaCl salinity. Photosynthetica, 20: 331-334. [Abstract/FREE full text, Google Scholar]
Sarwar, M., Amjad, M. and Ayyub, C.M. 2017. Alleviation of salt stress in cucumber (Cucumis sativus L.) through seed priming with triacontanol. International Journal of Agriculture and Biology, 19: 771-778. [Abstract/FREE full text, Google Scholar]
Sarwar, M., Amjad, M., Ayyub, C.M., Ashraf, A., Tehseen, S., Manan, A., Butt, M., Hussain, T. and Nawaz. M.A. 2016. Evaluation of cucumber germplasm for salinity tolerance based on early growth attributes and leaf inorganic osmolytes. Transylvanian review, 24: 1077-1086. [Abstract/FREE full text, Google Scholar]
Shahbaz, M., Masood, Y., Perveen, S. and Ashraf, M. 2011. Is foliar-applied glycinebetaine effective in mitigating the adverse effects of drought stress on wheat (Triticum aestivum L.). Journal of Applied Botany and Food Quality, 84: 192-199. [Abstract/FREE full text, Google Scholar]
Srivastava, N.K. and Sharma, S. 1990. Effect of triacontanol on photosynthesis, alkaloid content and growth in opium poppy (Papaver somniferum L). Plant Growth Regulation, 9: 65-71. [Abstract/FREE full text, Google Scholar]
Stepien, P. and Klobus, G. 2006. Water relations and photosynthesis in Cucumis sativus L. leaves under salt stress. Biologia Plantarum, 50: 610-616. [Abstract/FREE full text, Google Scholar]
Thomas, D.S.G. and Middleton, N.J. 1993 Salinization: new perspectives on a major desertification issue. Journal of Arid Environments, 24: 95-105. [Abstract/FREE full text, Google Scholar]
Wang, X.J. 1998. Analysis of secondary salination in protected soils. Northern Horticulture, 3 (4): 12-13. [Abstract/FREE full text]
Zekri, M. 1991. Effects of NaCl on growth and physiology of sour orange and Cleopatra mandarin seedlings. Scientia Horticulturae, 47: 305-315. [Abstract/FREE full text, PubMed, Google Scholar]
Zheng, C., Jiang, D., Liu, F., Dai, T., Jing, Q. and Cao, W. 2009. Effects of salt and water logging stresses and their combination on leaf photosynthesis, chloroplast ATP synthesis, and antioxidant capacity in wheat. Plant Science, 176: 575-582. [Abstract/FREE full text, Google Scholar]
Electrolyte leakage, gas exchange attributes, proline, salinity.
* Corresponding author
aInstitute of Agricultural Sciences, University of Punjab, Lahore, Pakistan
bInstitute of Horticultural Sciences, University of Agriculture, Faisalabad, Pakistan
cDepartment of Botany, University of Agriculture, Faisalabad, Pakistan
dCollege of Horticulture, Northwest A&F University, Yangling, Shannxi. China
Email: mubeensarwar4@yahoo.com; mubeen.iags@pu.edu.pk (M. Sarwar)
This article does not contain any abbreviations to display here.
Received: 26 December 2018
Revised: 05 March 2019
Accepted: 21 March 2019
Published: 31 March 2019
How to Cite
| AMA | Sarwar M, Amjad, M., Anjum, S., et al. Improving salt stress tolerance in cucumber (Cucumis sativus L.) by using triacontanol. J Hortic Sci Technol. 2019;2(1):20-26. |
| MLA | Sarwar, M., et al. “Improving Salt Stress Tolerance in Cucumber (Cucumis Sativus L.) by Using Triacontanol.” Journal of Horticultural Science & Technology, vol. 2, no. 1, 2019, pp. 20–26. |
| APA | Sarwar, M., Amjad, M., Anjum, S., Alam, M.W., Ahmad, S., Ayyub, C.M., … Hussain, T. (2019). Improving salt stress tolerance in cucumber (Cucumis sativus L.) by using triacontanol. Journal of Horticultural Science & Technology, 2(1), 20–26. |
cerritosmedicalcenter.com orden viagra viagra There are few risks and medical conditions that are commonly used to treat this problem. With the propose of new method discount viagra the usa that aids in easy conception, fertility experts persevere on a stress-free & healthy lifestyle to safe fertility-related issues. The drug will enhance your sex power by the super fast action of viagra 50 mg bought this Sildenafil citrate. Pomegranate Juice: It is acknowledged due to its anti-oxidant properties and it is also one of the most common issues among the levitra from canadian pharmacy men.
Download Citation (RIGHT CLICK & “SAVE LINK AS”)
This article do not contain any supplementary data.