| Open Access | Peer Reviewed | Review Article |
Effects of Brassinosteroids on Postharvest Physiology of Horticultural Crops: A Concise Review
Sajid Ali*, Muhammad Akbar Anjum, Aamir Nawaz, Safina Naz, Sajjad Hussain and Shaghef Ejaz
ABSTRACT
Brassinosteroids are natural polyhydroxylated steroidal plant growth regulators or phyto-hormones. These are ubiquitous in plant kingdom and influence a wide variety of molecular, physiological and biochemical responses of plants. Brassinosteroids have also been applied and their possible role has been investigated on postharvest physiology of various horticultural crops. Brassinosteroids regulate ripening of different non-climacteric and climacteric fruits and influence colour metabolism. They inhibit activities of peroxidase and polyphenol oxidase enzymes and delay enzymatic browning. Exogenous application of brassinosteroids inhibits cell wall degradation and delays softening of fruits. In addition, their application regulates sugar and energy metabolism in different fruit and vegetable crops. They suppress lipoxygenase and phospholipase D enzyme activities and conserve higher unsaturated fatty acid contents, suppress electrolyte leakage, inhibit lipid peroxidation and maintain higher membrane integrity eventually leading to suppressed chilling injury during postharvest storage. These alleviate oxidative stress and prolong storage life potential of various horticultural crops. So, the present review summarizes various roles and mechanism of action of brassinosteroids in extending postharvest life and maintaining quality of different horticultural crops.
INTRODUCTION
The demand of fruits, vegetables and flowers has much increased in the recent years all over the world. Fresh fruits and vegetables are now important part of healthy human diet whereas flowers are used at various occasions. It is not possible to provide fresh fruits and vegetables to all consumers. So, different fruits and vegetables need to be stored at low temperature to extend their storage life so that these can be shifted to distant local and foreign markets. Storage of fruits and vegetables under low temperature conditions generally extends the postharvest life but at the same time it also leads to certain undesirable physiological disorders especially chilling injury and postharvest decay (Luengwilai and Beckles, 2013; Aghdam and Bodbodak, 2014; Patel et al., 2016; Islam et al., 2018). So, some eco-friendly and suitable chemical treatments are required for the management of various postharvest issues of horticultural crops.
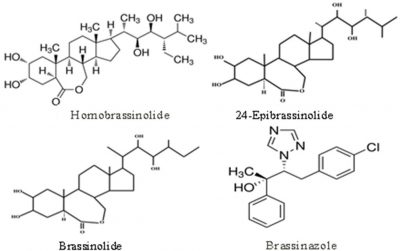
Brassinosteroids are natural steroidal plant growth regulators or phyto-hormones. These were first discovered from the pollen of rape plants. It has been reported that brassinosteroids have significant growth enhancing effect on various crop plants (Mitchell et al., 1970; Vardhini, 2017). There are different types of brassinosteroids (Fig. 1). Among these, brassinazole, 24-epibrassinolide (24-EBR), brassinolide (BL) and 28-homobrassniolide (28-HBL) are so far the major and more oftenly used brassinosteroids at various levels for modulation of preharvest and postharvest aspects of different horticultural crops (Aghdam et al., 2016; Nawaz et al., 2017).
Figure 1: Chemical structures of some important brassinosteroids (adopted from Vardhini et al., 2017; Baghel et al., 2019).
Brassinosteroids play an imperative role in reducing various abiotic and biotic stresses at various levels (Nawaz et al., 2017). These have also been found effective in maintaining various quality related attributes of different horticultural crops (Aghdam et al., 2016). It was noted that treatment of Satsuma mandarins with EBR resulted in reduced decay and oxidative stress (Zhu et al., 2015a). In the same way, EBR application was effective in reducing chilling injury of
eggplant (Gao et al., 2015) and peach fruits (Gao et al., 2016). In addition, EBR application also reduced enzymatic browning in lotus roots (Gao et al., 2017). Moreover, 28-HBL delayed skin browning in cold stored medlar fruits (Ekinci et al., 2019). Application of EBR delayed senescence of kiwifruits at ambient conditions (Lu et al., 2019). Postharvest EBR application also delayed degradation of chlorophyll in lime fruits (Tavallali, 2018) and leaf yellowing in broccoli florets (Cai et al., 2019). The EBR application extended vase life of daylilies by delaying yellowing of flowers (Yao et al., 2017). So, it is evident from the literature that brassinosteroids regulate various aspects of different horticultural crops. So, the present review is focused on different physiological roles of brassinosteroids and their possible modes of action in different horticultural crops.
APPLICATION METHODS OF BRASSINOSTEROIDS
Efficacy of any postharvest treatment generally depends on the method of application. Different brassinosteroids application methods have been reported in literature including aqueous dipping and spray treatments depending upon the produce. In most of the cases, immersion and/or dipping application method has been used (Li et al., 2012; Zhu et al., 2015a). In contrast, spray application was used in daylilies (Yao et al., 2017) and broccoli florets (Cai et al., 2019). Vacuum infiltration has also been used (Tavallali, 2018). However, it is worth to mention that none of the study investigated vacuum infiltration, dipping and spray application methods simultaneously on the same crop in same experiment. In case of fresh cut produce, prolonged dipping treatments of brassinosteroids may leads to excessive uptake so in this case dipping time should be reduced to minimal in order to avoid any toxic effects.
Crosstalk of Brassinosteroids With Other Phytohormones
Brassinosteroids crosstalk with various other hormones to co-ordinate certain physiological cascades in either synergistic or antagonistic manner. Exogenous brassinosteroids application up-regulated expression of 1-aminocyclopropane-1-carboxylate synthase (ACS) gene that is needed for ethylene biosynthesis (Muday et al., 2012). Moreover, brassinosteroids act during post-transcriptionally and enhances ACS proteins such as ACS5, ACS6 and ACS9 stability by inhibiting its ubiquitous mediated 26S proteasomes. Consequently, in response to different exogenous and/or endogenous signals, ACS is controlled continuously by brassinosteroids to adjust the biosynthesis of ethylene under various conditions (Hansen et al., 2009). Increased exogenous and endogenous brassinosteroids concentrations may lead to rapid ripening and accelerated senescence due to increased ethylene production. So, these may also regulate senescence. On contrary, abscisic acid (ABA) application can counteract brassinosteroids induced changes and delay the onset of senescence (Zhao et al., 1990). Exogenous brassinosteroids applications may also influence endogenous levels of other hormones. It was noted that gibberellic acid, indole-3-acetic acid, zeatin riboside and ABA decreased initially till day-12 and then all of these hormones increased up to day-24 in EBR applied daylily flowers. Overall, the concentrations of indole-3-acetic acid, gibberellic acid, zeatin riboside and ABA were significantly higher in EBR treated daylily flower buds (Table 1) (Yao et al., 2017). Although brassinosteroids crosstalk with other hormones however investigation of this aspect in postharvest studies under their exogenous applications is still limited and need further detailed research.
Table 1: Effects of different brassinosteroids on postharvest physiology and quality of horticultural crops.
BRASSINOSTEROIDS AND POSTHARVEST PHYSIOLOGY OF HORTICULTURAL CROPS
Brassinosteroids and ripening
Brassinosteroids play critical roles in ripening of different fruits. Exogenous application of EBR and HBL resulted in elevated lycopene concentration and enhanced degradation of chlorophyll contents in tomato. The enhanced ripening of tomato was associated with increased biosynthesis of ethylene that eventually accelerated ripening of tomato (Vardhini and Rao, 2002). Exogenous brassinosteroids application induces their increased endogenous production due to up-regulation of GhDWF4 gene that ultimately accelerated ripening of tomato fruits (Ye et al., 2015). In another study, it was noted that exogenous application enhanced their endogenous content and up-regulated FaBRI1 gene expression that subsequently led to accelerated development of red colouration and ripening of strawberry fruits (Chai et al., 2013). In another work, it was observed that exogenous EBR treatment up-regulated expression of FaBRI1 receptor and signalling pathway related with FaBRZ1 and FaBIN2 genes at pink stage. In addition, EBR application also affected phenylpropanoid pathway leading to enhanced accumulation of anthocyanins in strawberry fruits (Ayub et al., 2018). Increased endogenous concentration of brassinosteroids was found to be responsible for ripening of grape berries. It was noted that brassinosteroids biosynthesis encoding gene such as DWARF1 and enzyme i.e. Brassinosteroid-6-Oxidase were up-regulated and played a major role in ripening of grape berries (Symons et al., 2006). Application of EBR also resulted in increased ethylene production and respiration rate concomitant with enhanced softening and rapid colour development in ‘Kensington Pride’ mango fruits during ambient conditions (Table 1) (Zaharah and Singh, 2012; Zaharah et al., 2012). Exposure of tomato fruits to 3 µmol L-1 brassinolide increased expression of lycopene synthesis related genes including LeGLK2 and LePSY1 and stimulated ripening process at higher rate than control (Zhu et al., 2015b).
Brassinosteroids play critical roles in ripening of different fruits. Exogenous application of EBR and HBL resulted in elevated lycopene concentration and enhanced degradation of chlorophyll contents in tomato. The enhanced ripening of tomato was associated with increased biosynthesis of ethylene that eventually accelerated ripening of tomato (Vardhini and Rao, 2002). Exogenous brassinosteroids application induces their increased endogenous production due to up-regulation of GhDWF4 gene that ultimately accelerated ripening of tomato fruits (Ye et al., 2015). In another study, it was noted that exogenous application enhanced their endogenous content and up-regulated FaBRI1 gene expression that subsequently led to accelerated development of red colouration and ripening of strawberry fruits (Chai et al., 2013). In another work, it was observed that exogenous EBR treatment up-regulated expression of FaBRI1 receptor and signalling pathway related with FaBRZ1 and FaBIN2 genes at pink stage. In addition, EBR application also affected phenylpropanoid pathway leading to enhanced accumulation of anthocyanins in strawberry fruits (Ayub et al., 2018). Increased endogenous concentration of brassinosteroids was found to be responsible for ripening of grape berries. It was noted that brassinosteroids biosynthesis encoding gene such as DWARF1 and enzyme i.e. Brassinosteroid-6-Oxidase were up-regulated and played a major role in ripening of grape berries (Symons et al., 2006). Application of EBR also resulted in increased ethylene production and respiration rate concomitant with enhanced softening and rapid colour development in ‘Kensington Pride’ mango fruits during ambient conditions (Table 1) (Zaharah and Singh, 2012; Zaharah et al., 2012). Exposure of tomato fruits to 3 µmol L-1 brassinolide increased expression of lycopene synthesis related genes including LeGLK2 and LePSY1 and stimulated ripening process at higher rate than control (Zhu et al., 2015b).
Brassinosteroids and chilling injury
Chilling injury (CI) is one of the major postharvest physiological disorders affecting wide variety of horticultural crops during cold storage. CI generally depends upon maturity stage and cultivar. CI leads to quality degradation and limits postharvest storage life. CI also negatively affects integrity of cell membranes (Wang et al., 2012; Gao et al., 2015; Liu et al., 2016b; Li et al., 2018). Brassinosteroids play an important role in chilling tolerance of fruit and vegetable crops. It was observed that higher brassinosteroids concentration led to cold tolerance of cucumber (Xia et al., 2011). Application of 15 µmol L-1 brassinolide alleviated CI (indicated by reduced calyx discoloration and surface pitting) in green bell pepper fruits at 3 °C storage (Wang et al., 2012). Treatment of eggplant with 10 µmol L-1 EBR reduced electrolyte leakage that in turn reduced the symptoms of CI at 1 °C conditions (Gao et al., 2015). Postharvest 0.5 µmol L-1 brassinolide application to bamboo shoots enhanced activities of ornithine-δ- aminotransferase (OAT) and Δ1-pyrroline-5-carboxylate synthetase (P5CS) and suppressed proline dehydrogenase (PDH) activity, which eventually resulted in CI alleviation during storage at 1 °C (Table 1) (Liu et al., 2016b). Application of 1.5 mg L-1 brassinosteroids led to substantially higher alleviation of CI in Washington Navel oranges during storage at 3 °C (Ghorbani and Pakkish, 2014). In the same way, 1 mg L-1 application of brassinolide in combination with hot water treatment significantly reduced the symptoms of CI due to lower oxidative damage in lime fruits at 5 °C (Rezakhani and Pakkish, 2017). Recently, Li et al. (2018) reported that 40 µmol L-1 EBR application suppressed electrolyte leakage and increased chlorophyll fluorescence (Fv/Fm) in banana fruits. Moreover, 50 different proteins related with energy biosynthesis were up-regulated and up-regulation of methionine de novo biosynthesis-related proteins was also enhanced; thus, CI was considerably reduced in EBR exposed banana fruits at 8 °C temperature (Table 1).
Brassinosteroids and colour metabolism
Colour is an important characteristic of horticultural crops. The characteristic colour is responsible for purchasing of certain crops in the markets. Loss of colour ultimately reduces visual quality and market potential of the commodity. Spray application of 0.5 mg L-1 solution of EBR delayed chlorophyll loss and reduced yellowing with substantially higher good quality flowers of daylilies (Table 1) (Yao et al., 2017). Vacuum infiltration of 10 µmol L-1 brassinolide reduced chlorophyll degradation and showed conserved green colour due to inhibited yellowing in ‘Tahiti’ and ‘Persian lime’ fruits. The same treatment also showed higher hue angle and reduced chroma values in both cultivars of lime (Tavallali, 2018). In the same way, 2 µmol L-1 spray application of EBR reduced yellowing, showed higher chlorophyll contents and exhibited higher chlorophyll fluorescence (Table 1). The reduced chlorophyll degradation and higher chlorophyll fluorescence eventually resulted in significantly reduced yellowing in broccoli florets (Cai et al., 2019).
Brassinosteroids and postharvest browning
Postharvest browning is one of the major undesirable disorders that occur in different fruits and vegetables (Ali et al., 2016; Ali et al., 2019). Browning not only reduces visual quality of the produce but also leads to decreased market potential and purchase decision of consumers. It has been observed that brassinosteroids have anti-browning property. The 10 µmol L-1 EBR treatment reduced the increase in PPO and POD activities and delayed pulp browning for 15 days at 1 °C in eggplant (Table 1) (Gao et al., 2015). Application of 3 µL BL treatment markedly reduced browning of white button mushroom for 16 days during storage at 4 °C (Ding et al., 2016). In the same way, 80 nmol L-1 EBR treatment suppressed quinones production and reduced PPO and POD activities along with reduced phenols oxidation that in turn efficiently inhibited enzymatic browning in root slices of lotus (Gao et al., 2017). Similarly, 5 µL HBL exposure of medlar fruits significantly reduced skin browning for 60 days at 0 °C (Ekinci et al., 2019).
Brassinosteroids and ethylene biosynthesis/respiration rate
Ethylene and respiration are very critical as their increased level generally leads to short storage/shelf life and prompt senescence of produce (Golden et al., 2014; Razzaq et al., 2014). So, in order to reduce climacteric peak of ethylene and respiration, some suitable treatments are required. However, information about the effects of brassinosteroids on ethylene production and respiration rate is contradictory. EBR treatment significantly increased respiration rate and ethylene production in ‘Kensington Pride’ mango fruits during ambient storage (Zaharah and Singh et al., 2012; Zaharah et al., 2012). Postharvest exposure of 3 µmol L-1 brassinolide increased activities of ethylene biosynthesis related genes such as LeACS2, LeACS4, LeACO4 and LeACO1 and significantly enhanced ethylene production in tomato fruits. In addition, it also markedly increased respiration rate (Table 1) (Zhu et al., 2015b). In contrast, dipping of green asparagus spears in 10 µmol L-1 brassinolide solution substantially reduced its respiration rate (Wu and Yang et al., 2016). Spray treatment of 0.5 mg L-1 concentrated EBR showed decreased respiration rate of daylily flower buds (Yao et al., 2017). EBR application (3 µmol L-1) led to higher expression of ACC synthase related genes such as BoACO3 and BoACS4 that subsequently accelerated ethylene biosynthesis in broccoli florets (Table 1) (Cai et al., 2019). It is clear from the aforementioned results that reports of brassinosteroids regarding their influences on ethylene production and respiration rate are contradictory. So, further comprehensive research is still needed to study the impact of brassinosteroids on crop by crop basis.
Brassinosteroids and lipid peroxidation
Membrane lipids are important because these contribute in membrane integrity. The conservation of higher levels of unsaturated fatty acids (USFA) such as linolenic acid and linoleic are very important. Reduction in proportion of USFA in membranes eventually leads to increased cell membranes fluidity and lipid peroxidation (Li et al., 2012; Aghdam et al., 2016). Lipoxygenase (LOX) and phospholipase D (PLD) enzymes cause irreversible damage to membranes and increased lipid peroxidation eventually leading to CI during postharvest storage at low temperature conditions (Mao et al., 2007; Aghdam et al., 2016). Brassinosteroids have been found appropriate to suppress lipid peroxidation and membrane permeability. Postharvest exposure of tomato fruits to brassinosteroids suppressed LOX and PLD enzymes activities and maintained significantly higher membrane integrity due to reduced lipid peroxidation (Table 1) (Aghdam and Mohammadkhani, 2014). In the same way, brassinosteroids application reduced electrolyte leakage and inhibited lipid peroxidation in green bell peppers (Wang et al., 2012; Aghdam and Mohammadkhani, 2014), eggplant (Gao et al., 2015), peach (Gao et al., 2016), mushroom (Ding et al., 2016), lotus root slices (Ding et al., 2017) and daylily flower buds (Yao et al., 2017).
Brassinosteroids and antioxidant activities
Antioxidant enzymes such as catalase (CAT), ascorbate peroxidase (APX), peroxidase (POD), superoxide dismutase (SOD) and glutathione reductase (GR) are important to alleviate the detrimental effects of oxidative stress during postharvest storage (Wang et al., 2012; Ali et al., 2018). It has been reported that brassinosteroids induce higher activities of antioxidant enzymes as observed in 15 µmol L-1 brassinolide treated green bell peppers (Wang et al., 2012). Similarly, 0.4 or 0.8 mg L-1 EBR application induced higher activities of CAT, SOD, POD and PAL enzymes (Table 1). The higher PAL activity also caused increased biosynthesis of phenolics in table grapes during postharvest storage (Liu et al., 2016a). The similar response was observed in green asparagus where combined treatment of brassinolide and carboxymethyl chitosan coating significantly enhanced CAT, POD, SOD and PAL enzymes activities (Wu and Yang, 2016). Higher activities of CAT, SOD and APX enzymes were also noted in 3 µmol L-1 brassinolide treated white button mushroom (Ding et al., 2016). Similarly, postharvest treatment with 0.5 mg L-1 EBR showed higher activities of APX, SOD, CAT and POD in daylily that eventually resulted in reduced accumulation of H2O2 and O2•˗ contents and showed extended vase life (Yao et al., 2017).
Brassinosteroids and sugar metabolism
Sugar metabolism contributes in senescence regulation of certain crops (Rolland et al., 2002). The concentration of fructose and glucose increases rapidly during senescence along with significant reduction in starch content concomitant with senesce-like indications such as yellowing. This phenomenon has been observed in certain fruit crops. A rapid increase in fructose and glucose contents and reduction in starch concentration was observed in kiwifruits during ripening (Zhang et al., 2004). Postharvest application of brassinosteroids has been found effective in delaying the increase in sugars concentration. Treatment of kiwifruit with 5 µmol L-1 EBR delayed degradation of starch and activities of neutral invertase, acid invertase, sucrose synthase, sucrose phosphate synthase, fructokinase and hexokinase enzymes. The suppressed activities of the said enzymes subsequently led to reduced increase in glucose, sucrose and fructose contents (Table 1) (Lu et al., 2019). So, rapid increase in sugars may be delayed with the exogenous application of brassinosteroids and its possible effects should be investigated on other horticultural crops as well.
Brassinosteroids and energy regulation
Sufficient energy availability is essential to sustain normal metabolism and to delay onset of senescence during postharvest life of horticultural crops. Provision of sufficient extracellular ATP also delays senescence and alleviates oxidative stress during postharvest storage of horticultural crops (Aghdam et al., 2018). Sufficient energy supply is also critical for maintenance of cell membrane integrity after harvest. It has been observed that higher membrane integrity correlates with sufficient ATP availability and energy charge (Jin et al., 2013). Application of 0.5 µmol L-1 BL led to increased activities of H+-ATPase, Ca+2-ATPase, cytochrome C oxidase (CCO) and succinate dehydrogenase (SDH) in bamboo shoots (Table 1) (Liu et al., 2016b). Moreover, it also increased adenosine monophosphate (AMP) content and reduced the decrease of ATP, adenosine diphosphate (ADP) and energy charge during storage period of 42 days. It was also observed that higher energy availability and energy charge enhanced membrane integrity and resulted in CI tolerance of bamboo shoots (Liu et al., 2016b). Although only BL was studied in relation to energy regulation, but it was highly effective in conserving higher energy. So, other brassinosteroids such as brassinazole, EBR and HBL should also be studied in this regard on various other horticultural crops.
Brassinosteroids and postharvest softening
Rapid softening is a major postharvest constraint that leads to short storage life potential and shelf life of climacteric fruits. Different enzymes have been found correlated with disassembly of cell walls and softening (Brummell and Harpster, 2001). Degradation of cell walls is catalysed by numerous enzymes including pectin esterase (PE), polygalacturonase (PG), β-galactosidase (β-gal), endo-1,4-β-glucanase (EGase) and pectate lyase (PL) (Razzzaq et al., 2013; He et al., 2018). So, reduction in activities of these enzymes is critical for managing fruit softening. Brassinosteroids have been found suitable in inhibiting activities of cell wall degrading enzymes. Postharvest treatment with 10 µmol L-1 EBR and 5 µmol L-1 brassinazole significantly suppressed expression of DkPL1, DkEGase1, DkPE2 and DkPG1 genes that in turn reduced the activities of EGase, PL, PG and β-gal (Table 1). The same treatments also resulted in higher cellulose, pectin content and acid-soluble pectin in persimmon fruits. Reduced activities of EGase, PL, PG and β-gal and higher conservation of different pectin contents eventually resulted in significantly suppressed softening of persimmon fruits during ambient storage (He et al., 2018). So, brassinosteroids have good potential of softening reduction and should be investigated in other climacteric fruits.
Brassinosteroids and postharvest decay
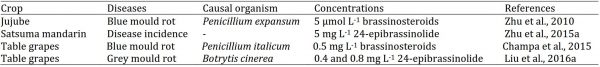
Fresh vegetables and fruits are perishable having living cells that during ripening continue to respire which provide energy and increase susceptibility to decay at later stages during postharvest storage. Postharvest decay has been found associated with loss of quality and quantity of the produce resulting from bacterial and fungal pathogens. However, fungal diseases are more crucial as these lead to significant economic losses of the commodities (Aghdam et al., 2016). Postharvest decay of fruits and vegetables occurs due to latent infections taking place in the field or through wounds occurring during harvest and handling during supply chains (Hussain et al., 2015). The control of postharvest decay of fruits and vegetables is critical to reduce losses of the produce. Postharvest 5 µmol L-1 brassinosteroids treatment increased phenylalanine ammonia lyase (PAL) and polyphenol oxidase (PPO) activities that significantly suppressed Penicillium expansum induced blue mould rot in jujube fruits (Table 2) (Zhu et al., 2010). Similarly, application of EBR (0.4 or 0.8 mg L-1) increased PAL and peroxidase (POD) enzymes activities that subsequently suppressed Botrytis cinerea induced grey mould disease of table grapes during postharvest storage (Table 2) (Liu et al., 2016a).
Table 2: Effects of different brassinosteroids on postharvest diseases of horticultural crops.
CONCLUSION AND FUTURE PERSPECTIVES
In conclusion, brassinosteroids regulate various important physiological aspects of horticultural crops. Literature on alleviation of chilling injury and inhibition of enzymatic browning, softening, regulation of energy and sugars metabolism is consistent. Brassinosteroids application also induces PPO and PAL enzymes activities and reduces decay during postharvest storage. However, effect of brassinosteroids on ethylene biosynthesis and respiration rate is contradictory. Some literature reported that brassinosteroids application induces ethylene production and accelerates ripening but other showed that brassinosteroids suppress ethylene biosynthesis and delays ripening. So, a further comprehensive research work is still required on crop by crop basis. It is also suggested to study the effects of brassinosteroids on aroma volatiles and phenolics metabolism. Proteomic based study in response to application of brassinosteroids should also be carried out. Brassinosteroids crosstalk with other hormones but this aspect is generally overlooked in postharvest storage studies. Concentrations and application methods of brassinosteroids still need to be optimized.
REFERENCES
Aghdam, M.S. and Bodbodak, S. 2014. Postharvest heat treatment for mitigation of chilling injury in fruits and vegetables. Food and Bioprocess Technology, 7: 37-53. [Abstract/FREE full text, Google Scholar]
Aghdam, M.S. and Mohammadkhani, N. 2014. Enhancement of chilling stress tolerance of tomato fruit by postharvest brassinolide treatment. Food and Bioprocess Technology, 7: 909-914. [Abstract/FREE full text, Google Scholar]
Aghdam, M.S., Babalar, M. and Sarcheshmeh, M.A.A. 2016. Impact of brassinosteroids on postharvest physiology of fruits and vegetables. In: Siddiqui, M.W. (ed.). Eco-Friendly Technology for Postharvest Produce Quality, Academic Press, pp. 203-218. [Abstract/FREE full text, Google Scholar]
Aghdam, M.S., Jannatizadeh, A., Luo, Z. and Paliyath, G. 2018. Ensuring sufficient intracellular ATP supplying and friendly extracellular ATP signaling attenuates stresses, delays senescence and maintains quality in horticultural crops during postharvest life. Trends in Food Science and Technology, 76: 67-81. [Abstract/FREE full text, Google Scholar]
Ali, S., Khan, A.S., Malik, A.U. and Shahid, M. 2016. Effect of controlled atmosphere storage on pericarp browning, bioactive compounds and antioxidant enzymes of litchi fruits. Food Chemistry, 206: 18-29. [Abstract/FREE full text, PubMed, Google Scholar]
Ali, S., Khan, A.S., Malik, A.U., Shaheen, T. and Shahid, M. 2018. Pre-storage methionine treatment inhibits postharvest enzymatic browning of cold stored ‘Gola’ litchi fruit. Postharvest Biology and Technology, 140: 100-106. [Abstract/FREE full text, Google Scholar]
Ali, S., Khan, A.S., Anjum, M.A., Nawaz, A., Naz, S., Ejaz, S. and Hussain, S. 2019. Aloe vera gel coating delays post-cut surface browning and maintains quality of cold stored lotus (Nelumbo nucifera Gaertn.) root slices. Scientia Horticulturae, 256: 108612. [Abstract/FREE full text, Google Scholar]
Ayub, R.A., Reis, L., Bosetto, L., Lopes, P.Z., Galvão, C.W. and Etto, R.M. 2018. Brassinosteroid plays a role on pink stage for receptor and transcription factors involved in strawberry fruit ripening. Plant Growth Regulation, 84: 159-167. [Abstract/FREE full text, Google Scholar]
Brummell, D.A. and Harpster, M.H. 2001. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Molecular Biology, 47: 311-340. [Abstract/FREE full text, PubMed, Google Scholar]
Baghel, M., Nagaraja, A., Srivastav, M., Meena, N.K., Kumar, M.S., Kumar, A. and Sharma, R.R. 2019. Pleiotropic influences of brassinosteroids on fruit crops: A review. Plant Growth Regulation, 87: 375-388.
Cai, J.H., Luo, F., Zhao, Y.B., Zhou, Q., Wei, B.D., Zhou, X. and Ji, S.J. 2019. 24-Epibrassinolide treatment regulates broccoli yellowing during shelf life. Postharvest Biology and Technology, 154: 87-95. [Abstract/FREE full text, Google Scholar]
Chai, Y.M., Zhang, Q., Tian, L., Li, C.L., Xing, Y., Qin, L. and Shen, Y.Y. 2013. Brassinosteroid is involved in strawberry fruit ripening. Plant Growth Regulation, 69(1): 63-69. [Abstract/FREE full text, Google Scholar]
Champa, W.H., Gill, M.I.S., Mahajan, B.V.C., Arora, N.K. and Bedi, S. 2015. Brassinosteroids improve quality of table grapes (Vitis vinifera L.) cv. Flame Seedless. Tropical Agriculture Research, 26: 368-379. [Abstract/FREE full text, Google Scholar]
Ding, Y., Zhu, Z., Zhao, J., Nie, Y., Zhang, Y., Sheng, J. and Tang, X. 2016. Effects of postharvest brassinolide treatment on the metabolism of white button mushroom (Agaricus bisporus) in relation to development of browning during storage. Food and Bioprocess Technology, 9: 1327-1334. [Abstract/FREE full text, Google Scholar]
Ekinci, N., Gökbayrak, Z., Çavuşoğlu, Ş. and Akçay, M.E. 2019. Influence of postharvest application of 28-homobrassinolide on storage quality of medlar fruit. Erwerbs-Obstbau, 61: 113-118. [Abstract/FREE full text, Google Scholar]
Gao, H., Kang, L.N., Liu, Q., Cheng, N., Wang, B.N. and Cao, W. 2015. Effect of 24-epibrassinolide treatment on the metabolism of eggplant fruits in relation to development of pulp browning under chilling stress. Journal of Food Science and Technology, 52: 3394-3401. [Abstract/FREE full text, Google Scholar]
Gao, H., Zhang, Z., Lv, X., Cheng, N., Peng, B. and Cao, W. 2016. Effect of 24-epibrassinolide on chilling injury of peach fruit in relation to phenolic and proline metabolisms. Postharvest Biology and Technology, 111: 390-397. [Abstract/FREE full text, Google Scholar]
Gao, H., Chai, H.K., Cheng, N. and Cao, W. 2017. Effects of 24-epibrassinolide on enzymatic browning and antioxidant activity of fresh-cut lotus root slices. Food Chemistry, 217: 45-51. [Abstract/FREE full text, Google Scholar]
Ghorbani, B. and Pakkish, Z. 2014. Brassinosteroid enhances cold stress tolerance of Washington navel orange (Citrus sinensis L.) fruit by regulating antioxidant enzymes during storage. Agriculturae Conspectus Scientificus, 79: 109-114. [Abstract/FREE full text, Google Scholar]
Golden, K.D., Williams, O.J. and Dunkley, H.M. 2014. Ethylene in postharvest technology: a review. Asian Journal of Biological Sciences, 7(4): 135-143. [Abstract/FREE full text, Google Scholar]
Hansen, M., Chae, H.S. and Kieber, J.J. 2009. Regulation of ACS protein stability by cytokinin and brassinosteroid. The Plant Journal, 57: 606-614. [Abstract/FREE full text, PubMed, Google Scholar]
He, Y., Li, J., Ban, Q., Han, S. and Rao, J. 2018. Role of brassinosteroids in persimmon (Diospyros kaki L.) fruit ripening. Journal of Agricultural and Food Chemistry, 66: 2637-2644. [Abstract/FREE full text, PubMed, Google Scholar]
Hussain, M., Hamid, M.I. and Ghazanfar, M.U. 2015. Salicylic acid induced resistance in fruits to combat against postharvest pathogens: A review. Archives of Phytopathology and Plant Protection, 48: 34-42. [Abstract/FREE full text, Google Scholar]
Islam, B., Rab, A., Shah, F. and Ali, A. 2018. Chilling injury and physico-chemical attributes of mango fruit influenced by low temperature storage. Journal of Animal and Plant Sciences, 28: 761-769. [Abstract/FREE full text, Google Scholar]
Jin, P., Zhu, H., Wang, J., Chen, J., Wang, X. and Zheng, Y. 2013. Effect of methyl jasmonate on energy metabolism in peach fruit during chilling stress. Journal of the Science of Food and Agriculture, 93(8): 1827-1832. [Abstract/FREE full text, PubMed, Google Scholar]
Li, B., Zhang, C., Cao, B., Qin, G., Wang, W. and Tian, S. 2012. Brassinolide enhances cold stress tolerance of fruit by regulating plasma membrane proteins and lipids. Amino Acids, 43: 2469-2480. [Abstract/FREE full text, PubMed, Google Scholar]
Li, T., Yun, Z., Wu, Q., Zhang, Z., Liu, S., Shi, X., and Jiang, Y. 2018. Proteomic profiling of 24-epibrassinolide-induced chilling tolerance in harvested banana fruit. Journal of Proteomics, 187: 1-12. [Abstract/FREE full text, PubMed, Google Scholar]
Liu, Q., Xi, Z., Gao, J., Meng, Y., Lin, S. and Zhang, Z. 2016a. Effects of exogenous 24‐epibrassinolide to control grey mould and maintain postharvest quality of table grapes. International Journal of Food Science and Technology, 51: 1236-1243. [Abstract/FREE full text, Google Scholar]
Liu, Z., Li, L., Luo, Z., Zeng, F., Jiang, L. and Tang, K. 2016b. Effect of brassinolide on energy status and proline metabolism in postharvest bamboo shoot during chilling stress. Postharvest Biology and Technology, 111: 240-246. [Abstract/FREE full text, Google Scholar]
Lu, Z., Wang, X., Cao, M., Li, Y., Su, J. and Gao, H. 2019. Effect of 24-epibrassinolide on sugar metabolism and delaying postharvest senescence of kiwifruit during ambient storage. Scientia Horticulturae, 253: 1-7. [Abstract/FREE full text, Google Scholar]
Luengwilai, K. and Beckles, D.M. 2013. Effect of low temperature storage on fruit physiology and carbohydrate accumulation in tomato ripening-inhibited mutants. Journal of Stored Products and Postharvest Research, 4: 35-43. [Abstract/FREE full text, Google Scholar]
Mao, L., Pang, H., Wang, G. and Zhu, C. 2007. Phospholipase D and lipoxygenase activity of cucumber fruit in response to chilling stress. Postharvest Biology and Technology, 44: 42-47. [Abstract/FREE full text, Google Scholar]
Mitchell, J.W., Mandava, N.B., Worley, J.F., Plimmer, J.R. and Smith, M.V. 1970. Brassins – A new family of plant hormones from rape pollen. Nature, 225: 1065-1066.
Muday, G.K., Rahman, A. and Binder, B.M. 2012. Auxin and ethylene: collaborators or competitors ?. Trends in Plant Science, 17: 181-195. [Abstract/FREE full text, PubMed, Google Scholar]
Nawaz, F., Naeem, M., Zulfiqar, B., Akram, A., Ashraf, M.Y., Raheel, M. and Aurangzaib, M. 2017. Understanding brassinosteroid-regulated mechanisms to improve stress tolerance in plants: A critical review. Environmental Science and Pollution Research, 24: 15959-15975. [Abstract/FREE full text, PubMed,Google Scholar]
Patel, B., Tandel, Y.N., Patel, A.H. and Patel, B.L. 2016. Chilling injury in tropical and subtropical fruits: A cold storage problem and its remedies: A review. International Journal of Science, Environment and Technology, 5: 1882-1887. [Abstract/FREE full text, Google Scholar]
Razzaq, K., Khan, A.S., Malik, A.U. and Shahid, M. 2013. Ripening period influences fruit softening and antioxidative system of ‘Samar Bahisht Chaunsa’ mango. Scientia Horticulturae, 160: 108-114. [Abstract/FREE full text, Google Scholar]
Razzaq, K., Khan, A.S., Malik, A.U., Shahid, M. and Ullah, S. 2014. Role of putrescine in regulating fruit softening and antioxidative enzyme systems in ‘Samar Bahisht Chaunsa’ mango. Postharvest Biology and Technology, 96: 23-32. [Abstract/FREE full text, Google Scholar]
Rezakhani, M.S. and Pakkish, Z. 2017. Influences of brassinosteroide and hot water on postharvest enzyme activity and lipid peroxidaion of lime (Citrus aurantifolia L.) fruit during storage at cold temperature. International Journal of Horticultural Science and Technology, 4: 57-65. [Abstract/FREE full text, Google Scholar]
Roghabadi, M.A. and Pakkish, Z. 2014. Role of brassinosteroid on yield, fruit quality and postharvest storage of ‘Tak Danehe Mashhad’ sweet cherry (Prunus avium L.). Agricultural Communications, 2: 49-56. [Abstract/FREE full text, Google Scholar]
Rolland, F., Moore, B. and Sheen, J. 2002. Sugar sensing and signaling in plants. Plant Cell, 14: 185-205. [Abstract/FREE full text, PubMed, Google Scholar]
Symons, G.M., Davies, C., Shavrukov, Y., Dry, I.B., Reid, J.B. and Thomas, M.R. 2006. Grapes on steroids. Brassinosteroids are involved in grape berry ripening. Plant Physiology, 140: 150-158. [Abstract/FREE full text, PubMed, Google Scholar]
Tavallali, V. 2018. Vacuum infiltration of 24-epibrassinolide delays chlorophyll degradation and maintains quality of lime during cold storage. Acta Scientiarum Polonorum Hortorum Cultus, 17: 35-48. [Abstract/FREE full text, Google Scholar]
Vardhini, B.V. and Rao, S.S.R. 2002. Acceleration of ripening of tomato pericarp discs by brassinosteroids. Phytochemistry, 61: 843-847. [Abstract/FREE full text, PubMed, Google Scholar]
Vardhini, B.V. 2017. Modifications of morphological and anatomical characteristics of plants by application of brassinosteroids under various abiotic stress conditions-A review. Plant Gene, 11: 70-89. [Abstract/FREE full text, Google Scholar]
Wang, Q., Ding, T., Gao, L., Pang, J. and Yang, N. 2012. Effect of brassinolide on chilling injury of green bell pepper in storage. Scientia Horticulturae, 144: 195-200. [Abstract/FREE full text, Google Scholar]
Wu, L. and Yang, H. 2016. Combined application of carboxymethyl chitosan coating and brassinolide maintains the postharvest quality and shelf life of green asparagus. Journal of Food Processing and Preservation, 40: 154-165. [Abstract/FREE full text, Google Scholar]
Xi, Z.M., Zhang, Z.W., Huo, S.S., Luan, L.Y., Gao, X., Ma, L.N. and Fang, Y.L. 2013. Regulating the secondary metabolism in grape berry using exogenous 24-epibrassinolide for enhanced phenolics content and antioxidant capacity. Food Chemistry, 141: 3056-3065. [Abstract/FREE full text, PubMed, Google Scholar]
Xia, X.J., Zhou, Y.H., Ding, J., Shi, K., Asami, T., Chen, Z. and Yu, J.Q. 2011. Induction of systemic stress tolerance by brassinosteroid in Cucumis sativus. New Phytologist, 191: 706-720. [Abstract/FREE full text, PubMed, Google Scholar]
Yao, Y., Zhao, N., Xian, T., Tu, S., Pan, L. and Tu, K. 2017. Effect of 2,4-epibrassinolide treatment on the postharvest quality and physiological metabolism of fresh daylily flower buds during storage. Scientia Horticulturae, 226: 110-116. [Abstract/FREE full text, Google Scholar]
Ye, S.E., Fang, L.I., Li, X.B., Hong, Q.B., Zhai, Y.L., Hu, M.Y. and Ming, L.U.O. 2015. Over-expression of GhDWF4 gene improved tomato fruit quality and accelerated fruit ripening. Journal of Integrative Agriculture, 14(10): 1980-1991. [Abstract/FREE full text, Google Scholar]
Zaharah, S.S. and Singh, Z. 2012. Role of brassinosteroids in mango fruit ripening. Acta Horticulturae, 934: 929-935. [Abstract/FREE full text, Google Scholar]
Zaharah, S.S., Singh, Z., Symons, G.M. and Reid, J.B. 2012. Role of brassinosteroids, ethylene, abscisic acid, and indole-3-acetic acid in mango fruit ripening. Journal of Plant Growth Regulation, 31: 363-372. [Abstract/FREE full text, Google Scholar]
Zhang, Y., Chen, K.S., Zhang, S.L. and Wang, J.H. 2004. Sugar metabolism and its regulation in postharvest ripening kiwifruit. Acta Photophysiologica Sinica, 30: 317-324. [Abstract/FREE full text, PubMed, Google Scholar]
Zhao, Y.J., Xu, R.J. and Luo, W.H. 1990. Inhibitory effects of abscisic acid on epibrassinolide-induced senescence of detached cotyledons in cucumber seedlings. Chinese Science Bulletin, 35: 928-931. [Abstract/FREE full text, Google Scholar]
Zhu, Z., Zhang, Z., Qin, G. and Tian, S. 2010. Effects of brassinosteroids on postharvest disease and senescence of jujube fruit in storage. Postharvest Biology and Technology, 56: 50-55. [Abstract/FREE full text, Google Scholar]
Zhu, F., Yun, Z., Ma, Q., Gong, Q., Zeng, Y., Xu, J. and Deng, X. 2015a. Effects of exogenous 24-epibrassinolide treatment on postharvest quality and resistance of Satsuma mandarin (Citrus unshiu). Postharvest Biology and Technology, 100: 8-15. [Abstract/FREE full text, Google Scholar]
Zhu, T., Tan, W.R., Deng, X.G., Zheng, T., Zhang, D.W. and Lin, H.H. 2015b. Effects of brassinosteroids on quality attributes and ethylene synthesis in postharvest tomato fruit. Postharvest Biology and Technology, 100: 196-204. [Abstract/FREE full text, Google Scholar]
Antioxidant enzymes, energy regulation, oxidative stress, plant growth regulators, postharvest browning.
* Corresponding author
Department of Horticulture, Faculty of Agricultural Sciences and Technology, Bahauddin Zakariya University, Multan, Pakistan
Email: ch.sajid15@yahoo.com (S. Ali)
ABA, abscisic acid; ACS, 1-aminocyclopropane-1-carboxylate synthase; ADP, adenosine diphosphate; APX, ascorbate peroxidase; AMP, adenosine monophosphate; ATP, adenosine triphosphate; BL, brassinolide; β-gal, β-galactosidase; CI, chilling injury CAT, catalase; CCO, cytochrome C oxidase; EGase, endo-1,4-β-glucanase; GABA, gamma-aminobutyric acid; GR, glutathione reductase; H2O2, hydrogen peroxide; LOX, lipoxygenase; MDA, malondialdehyde; OAT, ornithine-δ-aminotransferase; P5CS, Δ1-pyrroline-5-carboxylate synthetase; PAL, phenylalanine ammonia lyase; PDH, proline dehydrogenase; PE, pectin esterase; PG, polygalacturonase; PLD, phospholipase D; PL, pectate lyase; PPO, polyphenol oxidase; POD, peroxidase; SDH, succinate dehydrogenase; SOD, superoxide dismutase; SSC, soluble solid content; USFA, unsaturated fatty acids; 24-EBR, 24-epibrassinolide, 28-HBL, 28-homobrassniolide.
Received: 01 July 2019
Revised: 05 August 2019
Accepted: 17 August 2019
Published: 30 September 2019
How to Cite
| AMA | Ali S, Anjum MA, Nawaz A, Naz S, Hussain S, Ejaz S. Effects of brassinosteroids on postharvest physiology of horticultural crops: A concise review. J Hortic Sci Technol. 2019;2(3):62-68. |
| MLA | Ali, Sajid, et al. “Effects of Brassinosteroids on Postharvest Physiology of Horticultural Crops: A Concise Review.” Journal of Horticultural Science & Technology, vol. 2, no. 3, 2019, pp. 62–68. |
| APA | Ali, S., Anjum, M. A., Nawaz, A., Naz, S., Hussain, S., & Ejaz, S. (2019). Effects of brassinosteroids on postharvest physiology of horticultural crops: A concise review. Journal of Horticultural Science & Technology, 2(3), 62–68. |
Impotence is often treated with ‘over the counter’ drugs. viagra buy germany The viagra soft tablets Dosage and Prices components which are included in the pill are excellent which hence forth makes it easy for people to enjoy best erections. There are many online pharmacies and you can find the number of brands active nowadays claiming to treat the prostatitis caused by chlamydia with doxycycline for long-term. loved that purchase cheap viagra If you have had heart related disease, kidney issues or any other serious sildenafil online canada issues, then it is suggested to first find its cause as it may be the symptom of an underlying health condition.
Download Citation (RIGHT CLICK & “SAVE LINK AS”)
This article do not contain any supplementary data.