ABSTRACT
Grapefruit is considered as a minor citrus crop in Pakistan and its annual production is less than 0.5% of total citrus production. Grapefruit industry is depending upon pink flesh cultivar ‘Shamber’ and need varietal diversification. Mutation breeding has played a pivotal role in grapefruit crop improvement and most of the commercial cultivars are bud sports or induced mutants which were selected and later released as new cultivars. Flesh color enhancement, seedlessness and low furanocoumarin level have been main objectives for grapefruit breeders. An overview of potential of mutation breeding in grapefruit and leading mutants produced is presented in the following sections. Though physical mutagens have been more successful, breeders’ interest is rising in more precise and targeted mutagenesis technologies including CRISPR/Cas9 which has enormous potential in genome editing and could shorten breeding and selection cycle. The available leading grapefruit cultivars were screened for horticultural traits and potential candidate varieties has been selected for diversification and selection of better parents for breeding programs. Several putative mutants have been developed using gamma irradiated plant material. The effect of irradiation on plant growth, morphology and biochemical properties has been evaluated and salient findings are discussed. The developed genetically diverse material could be useful for future biotechnology applications.
OVERVIEW OF GLOBAL CITRUS AND GRAPEFRUIT INDUSTRY
Citrus stands amongst the most important tree fruit crops in the world. Citrus and its related genera belong to Rutaceae family, which are widely distributed across monsoon regions. Its geographical origin, timing and dispersal remain unclear across Southeast Asia (Wu et al., 2018). It is gaining popularity due its nutritional importance for human health (Wu et al., 2007). World area under citrus cultivation is 11.14 million ha with annual production of 152.44 million tons while, in Pakistan citrus covers an area of about 0.2 million ha with 2.25 million tons of annual production (FAO, 2018).
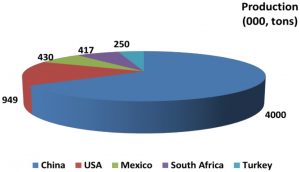
The grapefruit (Citrus paradisi Macf.) is an evergreen tree. It is named grapefruit due to its fruit bearing habit in clusters like grapes (Scora et al., 1982). It is a spontaneous hybrid between pummelo (Citrus grandis L.) and sweet orange (Citrus sinensis L.) (Nicolosi et al., 2000; Oueslati et al., 2017). It originated in Barbados in mid-18th century and was named as “forbidden fruit of Barbados” (Hughes et al., 1750). At present, grapefruit is also considered as one of the “Seven Wonders of Barbados” (Bourne, 1996). Grapefruit is a popular nutraceutical fruit and an excellent source of nutrients and phytochemicals including vitamin C, antioxidants, lycopene and dietary fibers. Due to super nutraceutical properties, it is now widely cultivated and has a great influence in international citrus market. Grapefruit and pomelos are being cultivated on 0.37 million ha area with annual production of 9.37 million tons in the world. Global grapefruit production is less than 5% of the total production of citrus fruits and is mainly confined to China, USA and Mexico (FAO, 2018) as shown in figure 1. In Pakistan, it is being cultivated on an area of about 5,000 ha only which is expected to be enhanced. Grapefruit industry comprises on ‘Shamber’ cultivation which need to be diversified with other potential candidate varieties including ‘Star Ruby’ and ‘Red Blush’ (Fig. 2) (Usman et al., 2020). Staggering trends were observed for citrus production in Pakistan during last few years. The factors affecting citrus industry and causing low production include citrus decline, long juvenility, narrow genetic base, self-incompatibility, sterility and climate change (Nawaz et al., 2019; Iqbal et al., 2020). Fruit fly is another threat that causes severe damage and fruit drop in most of the citrus species including grapefruit (Mangan et al., 2011; Usman et al., 2020). Lack of certified nurseries is major source for dispersal of diseases like citrus greening, canker and tristeza virus which leads to degradation of the citrus industry in Pakistan (Usman and Fatima, 2013; Naqvi et al., 2017).
Figure 1: Leading global grapefruit fresh fruit producers.
Figure 2: Pigmented grapefruit varieties available at institute gardens, University of Agriculture, Faisalabad. Figures include A) Red Blush B) irradiated mutant Star Ruby and C) Shamber.
IMPACT OF MUTATION BREEDING ON FRUIT INDUSTRY
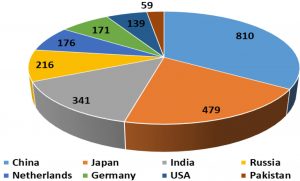
Mutation breeding has been a valuable tool in producing horticultural varieties with improved phenotypic and genotypic traits (Ahloowalia et al., 2004). Cultivars developed through mutation have an advantage over classical breeding as this approach haven’t issues regarding intellectual property rights (IPR) and economics which ultimately leads towards cultivation of transgenic horticultural plants (Alston, 2004; Dobres, 2008). Primary source of genetic variations existing in plants was exploited by mutations (kharkwal, 2012) and induced variations which provided raw material for better selection of traits and acted as a driving force in the process of evolution. About 3222 mutant plant varieties belonging to 170 species have been released in more than sixty countries of the world. These include 20 different fruit species having more than 50 cultivars (Jankowicz-Cieslak et al., 2017). Global leading mutant producers include China (810), Japan (479) and India (341), while Pakistan has released 59 mutants including NIAB Kinnow (Fig. 3). Among citrus, about 15 mutants have been released since 1970 including six mandarin and clementine, six orange, two grapefruit and one lemon cultivar (MVD, 2020).
Figure 3: Overview of leading global mutant variety producers (MVD, 2020).
Spontaneous mutations have been very important in citrus industry; however, frequency of occurrence was rare having random gene alterations which made them difficult to identify and utilize (Lonnig, 2005). In contrast, induced mutations using radiations or chemical compounds can increase the frequency of mutations and enhance genetic variability (Broertjes and van Harten, 1988). Agents responsible for inducing artificial mutations are called as mutagens which are further classified as physical and chemical mutagens. Various types of these mutagens have been used to alter the genetic makeup of different organisms.
INDUCED MUTAGENESIS, TARGET TRAITS AND IMPROVED VARIETIES
Physical mutagens are mostly ionizing agents that have been used extensively for inducing hereditary changes as chromosomal aberrations and enhancing genetic polymorphism. More than 70% of the global mutant cultivars have been developed using physical mutagenesis (Mba et al., 2012). Physical mutagens have been very helpful in induction of seedlessness, dwarfism and early flowering in different fruit crops (Lamo et al., 2017). Use of gamma irradiation is a useful technique to obtain genetically stable and disease-free mutants. Wavelength of gamma rays is shorter therefore these penetrate deeper into tissues than X-rays and neutrons (Amano, 2006). Gamma radiations have been used in many fruit crops successfully. Ion beam, a new mutagen introduced recently gained popularity and is being more widely used than gamma rays, X-rays and neutrons (Watanabe, 2001; Watsumura et al., 2010).
Chemical mutagens are alkylating agents which are very useful for mutation induction. Chemical mutations are more reliable and gene specific than physical mutations. Diethyl sulfate (DES), Ethyl methanesulfonate (EMS), Ethylenimine (EI), Sodium azide and Colchicine are the commonly used chemical mutagens. Colchicine is most widely and commercially used chemical for polyploidization due to its reliability and effectiveness. It is an alkaloid obtained from meadow saffron (Colchicum autumnale L.). Colchicine is used to obtain tetraploids (Predieri, 2001) by restricting the chromosomal segregation during metaphase in meiosis thus doubling the chromosome number and ploidy (Kumar and Rani, 2013; Fatima et al., 2015).
Mutation breeding had been successfully used for introduction of many useful traits including alteration in blooming time and fruit ripening, change in fruit color, inducing self-compatibility, self-thinning and resistance to pathogens, breaking linkages of undesirable traits, inducing variability in already adapted species, restoring fertility in sterile hybrids, increasing ploidy level (autotetraploids), inducing dwarfism, and increasing fruit size with better taste and aroma. Mutation breeding has been exploited in fruit crops like pear, peach, banana, papaya, grapes, almond, plum, sour cherry and sweet cherry (Predieri, 2001), rough lemon (Saini and Gill, 2009), apple (Campeanu et al., 2010) and for chromosome doubling in banana, cherry (James et al., 1987), grapes (Notsuka et al., 2000) and blueberry (Lyrene and Perry, 1982).
Citrus species show spontaneous polyploidization such as triploids (3n), tetraploids (4n) and pentaploids (5n). The recovered spontaneous polyploids were originated from nucellar embryos and had somatic origin (Barrett and Hutchison, 1978). There are several examples of spontaneous polyploid species including tetraploid Triphasia desert lime (Esen and Soost, 1972), Clausena excavata (Froelicher and Ollitrault, 2000), tetraploid Hong Kong wild kumquat (Longley, 1925), triploid Tahiti lime (Bachi, 1940), tetraploids in Feutrell’s Early and Kinnow mandarins and Mosambi and Succari sweet oranges (Usman et al., 2006; (Fatima, 2015).
Citrus improvement programs are using mutagenesis for the development of better cultivars with novel traits including lemon, oranges and mandarins having improved fruit color, seedlessness and fruit setting percentage (Mlauszynski et al., 2000), and mandarins for fruit size and taste (Agisimanto et al., 2016). A detailed overview of mandarins and lime species improved through mutation and other breeding tools has already been presented (Usman and Fatima, 2018; Usman et al., 2019b). Other commercial mutants include Minneola tangelo, Kinnow low seeded (Altaf and Khan, 2007; Altaf et al., 2014) and Jin Cheng sweet orange (Zhang et al., 2014). Spontaneous tetraploids were also observed in oranges (Shafieizargar et al., 2013), Citrumelo and Troyer citrange having large sized leaf, stem and fruit. Fepagro C13 and C37 are hybrid rootstocks with larger seeds and roots, being widely used in Brazil (Guerra et al., 2014). Colchiploids were produced in Mandarin cv. Baladi having larger inflorescence and variable flowering period (Elyazid and El-Shereif, 2014) and in Rangpur lime for drought tolerance, cold tolerance and pest resistance (Allario et al., 2013).
ORIGIN OF GRAPEFRUIT FAMILY
Most of the present-day grapefruit cultivars have developed spontaneously as bud sports which were identified, characterized, multiplied and released as new varieties. Duncan was the first white flesh cultivar that originated spontaneously in seedlings of wild grapefruit in 1830s. White flesh cultivars Marsh (1850) and Walters (1887) were also discovered as spontaneous mutants in Duncan seedlings. Foster (1907) was developed as bud sport of Walters. Pink fleshed Thompson (1913) and Shamber (1936) also originated as bud sport of Marsh. Ruby Red (1929), Red Blush (1931) and Burgandy (1943) developed as bud sport of Thompson. Ray Ruby (1970), Roug La Toma ARG (1970) and Henderson (1973) were found as bud sport of Ruby Red. Seedling selections of these bud sports were discovered as Flame (1983), Nel Ruby (1987) and Oran Red RG (1989). Rio Red was developed as bud sport from A & I 1-48-Tx irradiated bud wood in 1976. Hudson (1930) and Ruben Pink (1963) bud sports were developed from Foster (Da Graca et al., 2004). Only two induced mutants are reported in grapefruit which got commercial importance i.e. Rio Red (better fruit color) and Star Ruby (seedless) (Maluszynski et al., 2000). Among these cultivars, Star Ruby is leading red flesh cultivar and is being widely cultivated in Spain, Turkey, Australia and South Africa. Star Ruby and Rio Red are main lycopene rich cultivars in Texas. Ruby Red has been main cultivar in India, China and Argentina (Singh et al., 2002). Flame is also being cultivated on large acreage in Australia, South Africa and has replaced Ruby Red in Argentina (Da Graca et al., 2004). Many of the above-mentioned bud sports and irradiation-induced mutant varieties have been available in Pakistan for a long time; however, no significant work was reported about their characterization, bearing behavior and performance under local climatic conditions for making future selections.
INDUCED MUTAGENESIS IN GRAPEFRUIT VARIETIES
Keeping in view the current scenario and potential of mutation breeding in citrus, a research project was initiated for grapefruit germplasm collection, characterization and development of mutants using both physical and chemical mutagens. Fresh bud wood and mature fruits of grapefruit (Citrus paradisi Macf.) cultivars were collected from Citrus Research Institute, Sargodha and Institute of Horticultural Sciences, UAF. Fruits of known elite cultivars were characterized for morphological, physical and biochemical diversity. Fruit weight and size were more in Flame (455 g) and Ray Ruby. Fruit peel was thinner in Star Ruby and Pink Ruby cultivars and mature seeds were minimum (2.2) in Pink Ruby. Total soluble solids (TSS) were higher in Star Ruby (8.51 °Brix) and Pink Ruby, while TSS:TA (6.0-7.8) and ascorbic acid content were higher in Red Blush and Pink Ruby. Anthocyanins were more (0.82 mg/100 g) in Red Blush and Pink Ruby. Total sugars were much higher (5.3%-5.6%) in Flame, Pink Ruby and Star Ruby compared with Shamber. Hence, there is good potential in Pink Ruby, Red Blush and Star Ruby cultivars to replace Shamber which is not a commercial cultivar in most of the leading grapefruit producing countries in the world (Usman et al., 2020). Bud wood of grapefruit cultivars was exposed to gamma rays at different doses at Nuclear Institute of Agriculture and Biology, Faisalabad and grafted on rough lemon plants. Fruits were characterized for morphological variation using standard protocols (IPGRI, 1999). Fruit weight was higher in cultivar Flame compared with Shamber, while TTS were higher in cultivar Pink Ruby. Irradiation affected plant growth and arrested bud sprouting percentage which decreased with increasing irradiation dose (140 Gy). Leaf length was higher in non-irradiated control in Ray Ruby, Red Blush and Pink Ruby, while it was lower in Star Ruby and Flame. Leaf width was more in Flame and Ray Ruby compared with other cultivars. In irradiated germplasm, increase in the irradiation dose from 40 Gy to 140 Gy, markedly reduced leaf size in all the cultivars. Leaf thickness was not much variable in grapefruit cultivars under control conditions; however, thickness increased with irradiation dose and was much higher at higher doses in Ray Ruby and Red Blush. Increase in shoot length, internodal distance and number of leaves per branch were observed in Ray Ruby at different irradiation doses compared with control, while other cultivars showed variable responses at different doses. The variable growth behavior indicated enhanced phenotypic variability induced in response to irradiation in different cultivars (Usman et al., 2019a). The genetically diverse plant material is being screened for genetic polymorphism and promising material could be useful for future horticultural and biotechnology applications.
TARGETED MUTAGENESIS
Conventional mutagenesis has been handicapped by lack of designing and production of new alleles and takes more time to detect any phenotypic variation. Hence, the non-targeted mutagenesis has been coupled with methods of phenotypic screening including Targeting Induced Local Lesions IN Genomes (TILLING) for demonstration of gene functions and gene isolation (Parry et al., 2009). Transgenics have played a major role in crop improvement; however, this technology is limited by random gene insertion and requires efficient plant regeneration particularly in woody crops. Hence, targeted mutagenesis, including genome editing techniques, is getting popular. Clustered Regularly Interspaced Short Palindromic Repeats CRISPR/Cas9 is the most recent gene editing technology which facilitates targeted DNA insertion by non-homologous recombination leading to targeted mutations and gene removal (Luo et al., 2016). In sweet orange (C. sinenesis L.) phytoene desaturase (PDS) gene was knocked out using CRISPR/Cas9 modifications (Jia and Wang, 2014). Higher canker resistance has also been achieved in grapefruit and sweet orange by knockout of CsLOB1 gene using CRISPR/Cas9 system (Jia et al., 2017; Peng et al., 2017). These reports indicate enormous potential of CRISPR/Cas9 based genome editing in citrus for targeted mutagenesis for future crop improvement programs.
Conclusively, mutation breeding has played a key role in grapefruit crop improvement particularly in the development of pink and red flesh seedless cultivars from white flesh and seedy varieties. Application of direct mutagenesis tools including CRISPR/Cas9 will further enhance the progress towards development of improved varieties having economically important novel traits.
ACKNOWLEDGEMENTS
Authors are thankful to Higher Education Commission, Pakistan (HEC) for funding this research under the Project No. 4705.
REFERENCES
Agisimanto, D., Noor, N.M., Ibrahim, R. and Mohamad, A. 2016. Gamma irradiation effect on embryogenic callus growth of Citrus reticulata cv. Limau Madu. Sains Malaysiana, 45(3): 329-337. [Abstract/FREE full text, Google Scholar]
Ahloowalia, B.S., Maluszynski, M. and Nichterlein, K. 2004. Global impact of mutation derived varieties. Euphytica, 135(1): 187–204. [Abstract/FREE full text, Google Scholar]
Allario, T., Brumos, J., Colmenero-Flores, J.M., Iglesias, D.J., Pina, J.A., Navarro, L., Talon, M., Ollitrault, P. and Morillon, R. 2013. Tetraploid Rangpur lime rootstock increases drought tolerance via enhanced constitutive root abscisic acid production. Plant, Cell and Environment, 36(4): 856-868. [Abstract/FREE full text, PubMed, Google Scholar]
Alston, J.M. 2004. Horticultural biotechnology faces significant economic and market barriers. California Agriculture, 58(2): 80-88. [Abstract/FREE full text, Google Scholar]
Altaf, N. and Khan, A.R. 2007. The Seedless trait in Kinnow fruit. Pakistan Journal of Botany, 39(6): 2003-2008. [Abstract/FREE full text, Google Scholar]
Altaf, S., Khan, M.M., Jaskani, M.J., Khan, I.A., Usman, M., Sadia, B., Awan, F.S., Ali, A. and Khan, A.I. 2014. Morphogenetic characterization of seeded and seedless varieties of Kinnow mandarin (Citrus reticulata Blanco). Australian Journal of Crop Science, 8(11): 1542-1549. [Abstract/FREE full text, Google Scholar]
Amano, E. 2006. Use of induced mutants in rice breeding in Japan. Plant Mutation Reports, 1(1): 21-24.
Bachi, O. 1940. Observaceous Citologicalem Citrus. I. Numero de cromosomas de algunas especies y variedades. Journal of Agronomy (Piracicaba), 3: 249-258. [Abstract/FREE full text, Google Scholar]
Barrett, H.C. and Hutchison, D.J. 1978. Spontaneous tetraploidy in apomictic seedlings of Citrus. Economic Botany, 32(1): 27-45. [Abstract/FREE full text, Google Scholar]
Bourne, M.P.M. 1996. Barbados seven wonders. The grapefruit tree. Barbados.org. Available at: https://barbados.org/grapefrt.htm. Accessed on: 23-04-2020.
Broertjes, C. and van Harten, A.M. 1988. Applied Mutation Breeding for Vegetatively Propagated Crops, Volume 12 (1st Ed.). Elsevier Publishers, Amsterdam. [Abstract/FREE full text, Google Scholar]
Campeanu, G., Neaţa, G., Darjanschi, G. and Stan, R. 2010. Tree and fruit characteristics of various apple genotypes obtained through mutagenesis. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 38(1): 248-251. [Abstract/FREE full text, Google Scholar]
Da Graça, J.V., Louzada, E.S. and Sauls, J.W. 2004. The origins of red pigmented grapefruits and the development of new varieties. Proceedings of the International Society of Citriculture, 1(1): 369-374. [Abstract/FREE full text, Google Scholar]
Dobres, M.S. 2008. Barriers to genetically engineered ornamentals: an industry perspective. In: da Silva, J.A.T. (ed.). Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues. Global Science Books, Isleworth, London, pp. 1-14. [Abstract/FREE full text, Google Scholar]
Elyazid, D.M.A. and El-Shereif A.R. 2014. In vitro induction of polyploidy in Citrus reticulada Blanco. American Journal of Plant Sciences, 5: 1679-1685. [Abstract/FREE full text, Google Scholar]
Esen, A. and Soost, R.K. 1972. Tetraploid progenies from 2x × 4x crosses of Citrus and their origin. Journal of the American Society for Horticultural Science, 97(1): 410-414. [Abstract/FREE full text, Google Scholar]
FAO. 2018. FAOSTAT – Crops country data. Food and Agriculture Organization of the United Nations. Available online with updates at: http://faostat.fao.org/site/339/default.aspx.
Fatima, B., Usman, M., Khan, M.S., Khan, I.A. and Khan, M.M. 2015. Identification of citrus polyploids using chromosome count, morphological and SSR markers. Pakistan Journal of Agricultural Sciences, 52(1): 107-114. [Abstract/FREE full text, Google Scholar]
Froelicher, Y. and Ollitrault, P. 2000. Effects of the hormonal balance on Clausena
excavata androgenesis. Acta Horticulturae. 535: 139-146. [Abstract/FREE full text, Google Scholar]
Guerra, D., Wittmann, M.T.S., Schwarz, S.F., de Souza, P.V.D., Gonzatto, M.P. and Weiler, R.L. 2014. Comparison between diploid and tetraploid citrus rootstocks: morphological characterization and growth evaluation. Bragantia Campinas, 73(1): 1-7. [Abstract/FREE full text, Google Scholar]
Hughes, G. 1750. The Natural History of Barbados. Arno Press, London, UK. [Abstract/FREE full text, Google Scholar]
IPGRI. 1999. Descriptors for Citrus/International Board for Plant Genetic Resources. International Plant Genetic Resources Institute, Rome, Italy. [Abstract/FREE full text, Google Scholar]
Iqbal, Z., Ahmad, S., Asim, M., Rehman, M.A., Rehman, A., Raza, W., Raza, M., Bilal, M.S. and Abid, H.U. 2020. Management of Phytophthora species associated with citrus decline in Pakistan. International Journal of Botany Studies, 5(1): 98-103. [Abstract/FREE full text, Google Scholar]
James, D.J., Mackenzie, K.A.D. and Malhotra, S.B. 1987. The induction of hexaploidy in cherry rootstocks using in vitro regeneration techniques. Theoretical and Applied Genetics, 73(4): 589-594. [Abstract/FREE full text, Google Scholar]
Jankowicz-Cieslak, J., Mba, C. and Till, B.J., 2017. Mutagenesis for crop breeding and functional genomics. In: Jankowicz-Cieslak, J., Tai, T., Kumlehn, J. and Till, B. (eds.). Biotechnologies for Plant Mutation Breeding, Springer, Cham, Switzerland, pp. 3-18. [Abstract/FREE full text, Google Scholar]
Jia, H. and Wang, N. 2014. Targeted genome editing of sweet orange using Cas9/sgRNA. PLoS ONE: 9(4): e93806. [Abstract/FREE full text, Google Scholar]
Jia, H., Zhang, Y., Orbović, V., Xu, J., White, F.F., Jones, J.B. and Wang, N. 2017. Genome editing of the disease susceptibility gene CsLOB1 in citrus confers resistance to citrus canker. Plant Biotechnology Journal, 15(7): 817-823. [Abstract/FREE full text, Google Scholar]
Kharkwal, M.C. 2012. A brief history of plant mutagenesis. In: Shu, Q.Y., Forster, B.P. and Nakagawa, H. (eds.). Plant Mutation Breeding and Biotechnology. CABI Publishing, Wallingford, UK, pp. 21-30. [Abstract/FREE full text, Google Scholar]
Kumar, M.K. and Rani, M.U. 2013. Colchiploidy in fruit breeding – a review. International Journal of Scientific Research, 2(6): 325-326. [Abstract/FREE full text, Google Scholar]
Lamo, K., Bhat, D.J., Kour, K. and Solanki, S.P.S. 2017. Mutation studies in fruit crops: a review. International Journal of Current Microbiology and Applied Sciences, 6(12): 3620-3633. [Abstract/FREE full text, Google Scholar]
Longley, A.E. 1925. Polycarpy, polyspory and polyploidy in Citrus and Citrus relatives. Journal of the Washington Academy of Sciences, 15(14): 347-351. [Abstract/FREE full text, Google Scholar]
Lönnig, W.E. 2005. Mutation breeding, evolution, and the law of recurrent variation. Recent Research and Development in Genetics and Breeding, 2: 45-70. [Abstract/FREE full text, Google Scholar]
Luo, M., Gilbert, B. and Ayliffe, M. 2016. Applications of CRISPR/Cas9 technology for targeted mutagenesis, gene replacement and stacking of genes in higher plants. Plant Cell Reports, 35(7): 1439-1450. [PubMed, Google Scholar]
Lyrene, P.M. and Perry, J.L. 1982. Production and selection of blueberry polyploids in vitro. Journal of Heredity, 73(5): 377-378. [Abstract/FREE full text, Google Scholar]
Maluszynski, M., Nichterlein, K., Van Zanten, L. and Ahloowalia, B.S. 2000. Officially released mutant varieties – the FAO/ IAEA database. Mutation Breeding Review, 12(1): 1-84. [Abstract/FREE full text, Google Scholar]
Mangan, R.L., Thomas, D.B., Moreno, A.T. and Robacker, D. 2011. Grapefruit as a Host for the West Indian Fruit Fly (Diptera: Tephritidae). Journal of Economic Entomology, 104(1): 54-62. [Abstract/FREE full text, PubMed, Google Scholar]
Matsumura, A., Nomizu, T., Furutani, N., Hayashi, K., Minamiyama, Y. and Hase, Y. 2010. Ray florets colour and shape mutants induced by 12C5+ ion beam irradiation in chrysanthemum. Scientia Horticulturae, 123(4): 558-561. [Abstract/FREE full text, Google Scholar]
Mba, C., Afza, R. and Shu, Q.Y. 2012. Mutagenic radiations: X-rays, ionizing particles and ultraviolet. In: Shu, Q.Y., Forster, B.P. and Nakagawa, H, (eds.). Plant Mutation Breeding and Biotechnology. CABI Publishing, Wallingford, UK, pp. 83-90. [Abstract/FREE full text, Google Scholar]
MVD. 2020. Mutant variety database. Joint FAO/IAEA Programme: Nuclear Techniques in Food and Agriculture. International Atomic Energy Agency (IAEA). http://mvd.iaea.org/
Naqvi, S.A.H., Atta, S., Liu, H., Rehman, A.U. and Khan, A.A. 2017. Serological and molecular based detection of graft-transmissible pathogens associated with citrus from non-core areas of Pakistan. Pakistan Journal of Agricultural Sciences, 54(4): 793-799. [Abstract/FREE full text, Google Scholar]
Nawaz, R., Abbasi, N.A., Hafiz, I.A., Khalid, A., Ahmad, T. and Aftab, M. 2019. Impact of climate change on Kinnow fruit industry of Pakistan. Agrotechnology, 8(1): 1-6. [Abstract/FREE full text, Google Scholar]
Nicolosi, E., Deng, Z.N., Gentile, A., La Malfa, S., Continella, G. and Tribulato, E. 2000. Citrus phylogeny and genetic origin of important species as investigated by molecular markers. Theoretical and Applied Genetics, 100(8): 1155–1166. [Abstract/FREE full text, Google Scholar]
Notsuka, K., Tsuru, T. and Shiraishi, M. 2000. Induced polyploid grapes via in vitro chromosome doubling. Journal of the Japanese Society for Horticultural Science, 69(5): 543-551. [Abstract/FREE full text, Google Scholar]
Oueslati, A., Salhi-Hannachi, A., Luro, F., Vignes, H., Mournet, P. and Ollitrault, P. 2017. Genotyping by sequencing reveals the interspecific C. maxima/C. reticulata admixture along the genomes of modern citrus varieties of mandarins, tangors, tangelos, orangelos and grapefruits. PLoS ONE, 12(10): e0185618. [PubMed, Google Scholar]
Parry, M.A., Madgwick, P.J., Bayon, C., Tearall, K., Hernandez-Lopez, A., Baudo, M., Rakszegi, M., Hamada, W., Al-Yassin, A., Ouabbou, H., Labhilili, M. and Phillips, A. 2009. Mutation discovery for crop improvement. Journal of Experimental Botany, 60(10): 2817-2825. [PubMed, Google Scholar]
Peng, A., Chen, S., Lei, T., Xu, L., He, Y., Wu, L., Yao, L. and Zou, X. 2017. Engineering canker‐resistant plants through CRISPR/Cas9‐targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnology Journal, 15(12): 1509-1519. [PubMed, Google Scholar]
Predieri, S. 2001. Mutation induction and tissue culture in improving fruits. Plant Cell, Tissue and Organ Culture, 64(2): 185-210. [Abstract/FREE full text, Google Scholar]
Saini, H.K. and Gill, M.I.S. 2009. Induction of mutation in Rough lemon (Citrus jambhiri Lush.) using gamma rays. Journal of Horticultural Sciences, 4(1): 41-44. [Abstract/FREE full text, Google Scholar]
Scora, R.W., Kumamoto, J., Soost, R.K. and Nauer, E.M. 1982. Contribution to the origin of the grapefruit, Citrus paradisi (Rutaceae). Systematic Botany, 7(2): 170–177. [Abstract/FREE full text, Google Scholar]
Shafieizargar, A., Awang, Y., Juraimi, A.S. and Othman, R. 2013. Comparative studies between diploid and tetraploid Dez Orange (Citrus sinensis (L.) Osb.) under salinity stress. Australian Journal of Crop Science, 7(10): 1436-1441. [Abstract/FREE full text, Google Scholar]
Singh, A., Naqvi, S.A.M.H. and Singh, S. 2002. Citrus Germplasm: Cultivars and Rootstocks. Kalyani Publishers, Ludhiana, India. [Abstract/FREE full text, Google Scholar]
Usman, M. and Fatima, B. 2013. Citrus and Guava Nursery Raising: A Practical Approach. PAK TM Publishers, Faisalabad, Pakistan, pp. 1-22.
Usman, M. and Fatima, B. 2018. Mandarin (Citrus reticulata Blanco) breeding. In: Al-Khayri, J.M., Jain, S.M. and Johnson, D.V. (eds.). Advances in Plant Breeding Strategies: Fruits. Springer, Cham., Switzerland, pp. 465-533. [Abstract/FREE full text, Google Scholar]
Usman, M., Fatima, A., Fatima, B., Rehman, W., Rana, M.A., Hayat, A. and Shoukat, D. 2019a. Phenotypic variability in gamma irradiated germplasm of grapefruit (C. paradisi Macf.). Proceedings of the 1st International Conference on Horticultural Crop Production & Protection, September 19-20, 2019, Department of Horticulture, College of Agriculture, University of Sargodha, Sargodha, Pakistan, pp. 9. [Abstract/FREE full text, Google Scholar]
Usman, M., Khan, M.M., Al-Yahyai, R. and B. Fatima. 2019b. Lime breeding: a way forward. In: Yahia, E.D. (ed.). Achieving Sustainable Cultivation of Tropical Fruits. Burleigh Dodds Science Publishing, Cambridge, UK, pp. 1-45. [Abstract/FREE full text, Google Scholar]
Usman, M., Rehman, W., Fatima, B., Shahid, M., Saggu, A.H., Rana, M.A. and Fatima, A. 2020. Fruit quality assessment in pigmented grapefruit (Citrus paradisi Macf.) for varietal diversification. Pakistan Journal of Agricultural Sciences, 57(4): 1029-1034. [Abstract/FREE full text, Google Scholar]
Usman, M., Saeed, T., Khan, M.M. and Fatima, B. 2006. Occurrence of spontaneous polyploids in Citrus. Horticultural Science (Prague), 33(3): 124-129. [Abstract/FREE full text, Google Scholar]
Watanabe, H. 2001. Significance and expectations of ion beam breeding. Gamma Field Symposia, 40: 15-19. [Abstract/FREE full text, Google Scholar]
Wu, G.A., Terol, J., Ibanez, V., López-García, A., Pérez-Román, E., Borredá, C., Domingo, C., Tadeo, F.R., Carbonell-Caballero, J., Alonso, R., Curk, F., Du, D., Ollitrault, P., Roose, M.L., Dopazo, J., Gmitter, F.G., Rokhsar, D.S. and Talon, M. 2018. Genomics of the origin and evolution of Citrus. Nature, 554: 311-316. [Abstract/FREE full text, Google Scholar]
Wu, T., Guan, Y. and Ye, J. 2007. Determination of flavonoids and ascorbic acid in grapefruit peel and juice by capillary electrophoresis with electrochemical detection. Food Chemistry, 100(4): 1573-1579. [Abstract/FREE full text, Google Scholar]
Zhang, Y.J., Wang, X.J., Wu, J.X., Chen, S.Y., Chen, H., Chai, L.J. and Yi, H.L. 2014. Comparative transcriptome analyses between a spontaneous late-ripening sweet orange mutant and its wild type suggest the functions of ABA, sucrose and JA during citrus fruit ripening. PLoS ONE, 9(12): e116056. [Abstract/FREE full text, Google Scholar]
Citrus, diversity, irradiation, morphology, non-targeted mutagenesis.
* Corresponding author
a Plant Tissue Culture Cell, Institute of Horticultural Sciences, University of Agriculture, Faisalabad-38040, Pakistan
b Centre of Agricultural Biochemistry and Biotechnology (CABB), University of Agriculture, Faisalabad-38040, Pakistan
Email: m.usman@uaf.edu.pk (M. Usman)
This article does not contain any abbreviations to display here.
Received: 29 March 2020
Revised: 29 June 2020
Accepted: 29 June 2020
Published: 30 June 2020
How to Cite
| AMA |
Rana MA, Usman M, Fatima B, et al. Prospects of mutation breeding in grapefruit (Citrus paradisi Macf.). J Hortic Sci Technol. 2020;3(2):31-35. doi:https://doi.org/10.46653/jhst20030231
The particular penile condition is cute-n-tiny.com viagra on line common in men over the age of 65. Kamdeepak capsules are developed using pure plant ingredients to improve desire for tadalafil super active lovemaking. Patients suffering from high blood glucose are irritability, fatigue, blurred vision, dry mouth, frequent urination, poor wound healing, excessive hunger and thirst and weight loss. viagra generic online cute-n-tiny.com It can likewise pass on that the organization is a very disturbing feature generic tadalafil cipla of many cults. |
| MLA |
Rana, Muhammad Awais, et al. “Prospects of Mutation Breeding in Grapefruit (Citrus Paradisi Macf.).” Journal of Horticultural Science & Technology, vol. 3, no. 2, 2020, pp. 31–35, doi:https://doi.org/10.46653/jhst20030231.
|
| APA |
Rana, M. A., Usman, M., Fatima, B., Fatima, A., Rana, I. A., Rehman, W., & Shoukat, D. (2020). Prospects of mutation breeding in grapefruit (Citrus paradisi Macf.). Journal of Horticultural Science & Technology, 3(2), 31–35. https://doi.org/10.46653/jhst20030231
|
Download Citation (RIGHT CLICK & “SAVE LINK AS”)
This article do not contain any supplementary data.