ABSTRACT
Horticultural crops, being a prime source of essential nutrients, staple food and foreign exchange for a large part of human population, exhibit a unique status among growers and consumers. Ever increasing population across the world and changing climate conditions are badly affecting food security. Urgent needed improvement of production and enhanced adaptation to changing environmental conditions of horticultural crops seems unlikely to be met by conventional breeding technologies. Fortunately, one of the modern molecular techniques namely “clustered regularly interspaced short palindromic repeat (CRISPR)” technology has opened a new window to genetically improve these crops. Employing CRISPR technology, many crops for instance tomato, potato, watermelon and grapes have been successfully genetically engineered in order to improve their nutritional value and enhance adaptability towards changing climates. In the same way, resistance against many potential diseases have been developed modifying the genetics of certain horticultural crops. In this mini review, we have briefly discussed the successful CRISPR-Cas9 based studies conducted in horticultural crops and tried to present valuable source for the horticulturists working on biotic and abiotic stresses.
INTRODUCTION
Horticultural crops such as fruits, vegetables and ornamental plants are prime source of vitamins, minerals, carbohydrates, proteins, fats, energy, and economic returns. Farmers get lucrative income by selling these crops and the country may earn a handsome foreign exchange by exporting the surplus produce (Slavin and Lloyd, 2012). In past, the only approach to improve these crops in terms of nutritional value, taste, attractiveness, yield, and resistance to biotic and abiotic factors was conventional breeding (Ashraf, 2010; Tester and Langridge, 2010). However, conventional breeding, being time consuming and tedious in overall procedure compelled the breeders to think regarding better genetic improvement sources and techniques. Ultimately, this urge led researches to the discovery of several genome editing tools; for instance, Mega Nucleases, Zinc Finger Nucleases (ZFNs) and TALENS (transcription activator-like effecter nucleases) (Carroll, 2011; Mehfouz et al., 2011; Li et al., 2012). However, these techniques, were constantly replacing each other because of latter’s greater accuracy and efficacy comparative to the former’s one. In the meantime, 21th century gave rise to a novel genome editing technique that has not been exemplified before known as “CRISPR/Cas9 system” (Cong et al., 2013; Corte et al., 2019). Due to simplicity, efficacy and accuracy, this technique is largely popular among the scientists and is rated at the top among other genome editing strategies (Kartute et al., 2017; Corte et al., 2019). It is widely considered among breeders and scientists that the CRISPR/Cas9 mediated genome editing is capable to improve the quality and production of horticultural crops by incorporation and breeding of desired traits.
What is CRISPR/Cas9?
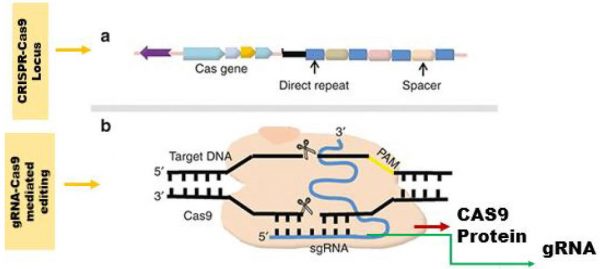
CRISPR stands for “Clustered regularly interspaced short palindrome repeats” while Cas9 is an enzyme that is encoded by a gene present very next to CRISPR region. Bacteria and archaea have evolved this immune system to defend themselves against viruses known as phages (Jiang and Dooudna, 2017). To hijack bacterial cell’s machinery for the replication of their own DNA, viruses constantly infect and inject their DNA into infected cells. When, for any reason, the viral DNA is cleaved prior to infection, resulting in small fragments, just for the sake of memory these are incorporated into bacterial genome loci called CRISPR. Thus, CRISPR is a small part of bacterial DNA that exhibits two distinct alternative short sequences of interest (Fig. 1a). First is repeated sequence (23 to 47 bps) of nucleotides that is present already in bacterial genome prior to viral infection (Makarova et al., 2011). While second is spacers sequences (21-72 bps) which are cleaved short fragments of viral DNA adjusted among repeated sequences (Makarova et al., 2011). Ultimately, spacers act as memories and enable bacteria to find viruses and fight for future attack.
Figure 1: Mechanism of CRISPR-Cas9-mediated genome editing. In part ‘a’ CRISPR-Cas9 locus consisting of Cas9 encoding gene, direct repeats and spacer sequences have been shown. While in part ‘b’ of the figure guider RNA (gRNA) based detection and Cas9 based editing of target DNA is presented.
CRISPR/Cas9 mechanism
CRISPR/Cas9 loci consist of two parts, the CRISPR (repeats and spacers) and Cas9. Firstly, CRISPR loci transcribes into Pre-crRNA and processed into crRNA (part of guide RNA) as described by Makarova et al. (2011). Each piece of crRNA consists of a single spacer between two half-repeats. Secondly, Cas9 loci transcribe and get translated into endonuclease enzyme known as Cas9 (Deltcheva et al., 2011). CRISPR/Cas9 jointly forms a complex and work together as a system. Upon the infection of virus whose DNA short fragments are already present in CRISPR loci is detected when CRISPR/Cas9 complex scans the viral DNA and forms complementary spacers-viral DNA pairing (Cong et al., 2013). In this way, after detection of virus, Cas9 protein cuts the viral DNA and ensures immunity to bacteria. Thus, it is understandable now that how CRISPR/Cas9 complex is used for genome editing in variety of organisms. In fact, scientists use guide RNA (around 20bp long) which consists of complementary sequence with the gene of interest. Further, Cas9 has ability to cut the DNA at the specific position in the genome (Fig. 1b). In this way, the guide RNA moves and finds the specific complementary sequence of DNA and gets bind (Jinek et al., 2012). This means only guide RNA bind to the target sequence. The Cas9 follows the guide RNA in the DNA sequence and make cut at both strands of target DNA at specific point. The cell recognizes the damage and tries to fix it (Jinek et al., 2012).
Use of CRISPR/Cas9 for vegetable crops
By implementing CRISPR/Cas9 system for the improvement of vegetable crop, several significant achievements have been made during the last few years. For instance, parthenocarpic tomato fruit, highly demanded in processing industry, has been developed by mutating SlAGL6 and SlIAA9 genes (klap et al., 2017). CRISPR/Cas9 based genetic engineering targeting Phytoene desaturase gene in watermelon has also been practiced (Tian et al., 2017). The CRISPR/Cas9 system has also been utilized for developing resistance against various diseases. Tomato genome has SlMlo1 gene that confers susceptibility to powdery mildew fungi (Oidium neolycopersici) (nekrasov et al., 2017). Hence, researchers targeted two active positions in SlMlo1 gene with the help of double sgRNA and successfully developed transgene-free powdery mildew-resistant tomato. RNA viruses use various components of plant cell such as translation initiation factor namely eIF4Efor their replication and multiplication (Nekrasov et al., 2017). Interestingly, eIF4E silencing in melon and tomato has led to the broad-spectrum RNA virus-resistance (Mazier et al., 2011; Rodriguez-Hernandez et al., 2012). In the same way, knocking out eIF4E gene from cucumber genome imparted broad spectrum resistance against potyviridae family viruses (Chandrasekaran et al., 2016). Another strategy to develop multiple virus resistance using CRISPR/Cas9 system in plants could be the introduction of gRNAs that targets multiple viruses along with single Cas9 gene. CRISPR-associated Cas9 enzymatic protein has also been implemented for the betterment of in quality vegetables. Starch quality in potato is considered valuable parameter in its consumption as food or any other industrial applications. Mutating GBSS (granule bound starch synthase) gene, the “waxy genotype” producing only amylopectin containing starch has been successfully developed in hexaploid potato (Andresson et al., 2017). Metabolism based genome editing in vegetables is another worthy field to improve their quantity and quality. However, targeting multiple genes involved in any metabolic pathway to improve nutritional value without any side effect is a challenging task.
Use of CRISPR/Cas9 for fruit crops
CRISPR-CAS9 is used to deploy the resistance against one of the devastating diseases in citrus known as citrus canker by knocking-out CsLOB1susceptible gene (Jia et al., 2017). According to a report, over expression of Arabidopsis thaliana NPRI produced sweet orange (Citrus sinensis Osbeck.) plants with enhanced resistance to Huanglongbing (HLB; citrus greening). Phloem expression of NRP1 was equally effective to impart resistance against HLB. These plants has a normal phenotypes and few transgenic lines remained disease free in the field up to 36 months under high disease pressure area (Dutt et al., 2015). Another group of scientists also developed resistance against the same disease by adopting different strategies. They reduced the expression of CsLOB1susceptible gene by targeting its promoter which is regulated by pathogen secreted effector namely PthA4 (Peng et al., 2017). In addition to citrus, CRISPR/Cas9 based genetic engineering has also been practiced in grapes. Scientists attempted the targeting of Phytoene desaturase gene in grapes (Nkajima et al., 2017). CRISPR/Cas9 mediated editing of TFL1 in apple and pear reduced juvenile period and induced early flowering, 91% of the apple transgenic lines and 9% of the pear lines exhibited early flowering (Charrier et al., 2019). Interestingly, several databases aimed to provide target sites for CRSIPR/Cas9-based genome editing are being developed. As an outcome of this effort one such database specifically for grape namely “Grape-CRISPR” has already been published (Wang et al., 2016). These databases will greatly assist researchers for the identification of target sites in a gene of interest.
Use of CRISPR-CAS9 for ornamentals and medicinal crops
CRISPR/Cas9-mediated genome editing is also practiced in ornamental plants. First of all, a model legume plant namely Lotus japonicas was subjected to genome editing. Several genes of this plant were targeted; for example, symbiosis receptor-like kinase (nitrogen fixation-related) encoding genes and leghemoglobin loci (LjLb1, LjLb2, andLjLb3) were targeted (Wang et al., 2016). Chrysanthemum morifolium is another hexaploid ornamental plant that has disclosed the application of CRISPR/Cas9. The transgenic Chrysanthemum morifolium plant expressing yellowish-green fluorescent protein (CpYGFP) gene was selected for genome editing. In this study, two sgRNAs were used to mutate the CpYGFP gene at two different positions (kishi-Kaboshi et al., 2017). Previously, it has found that chrysanthemum is a natural source of bio-insecticides (Kurkute et al., 2017). However, the pathways which are involved in the biosynthesis of these insecticides are still poorly understood. It is though that CRISPR/Cas9 based genome editing can lead to the manipulation of these biosynthetic pathways leading towards the formation of more efficient, and broad-spectrum insecticides (Kurkute et al., 2017). In the same way, Chinese medicinal plant (Salvia miltiorrhiza) has genetically been edited using CRISPR/Cas9 system. In this research work, a gene involved in tanshinone biosynthesis known as diterpene synthase gene (SmCPS1) was knocked-out and successfully tanshinones lacking plants were developed without affecting other phenolic metabolites (Li et al., 2017). In the same way, genome editing in medicinal plants can also help in studying the biosynthetic pathways of medicinally important compounds and ultimately their manufacturing at a commercial scale.
CONCLUSION
CRISPR/Cas9 genome editing system is a novel technique being used by the scientists to enhance production and improve quality of horticultural crops. A number of research groups around the globe are working on CRISPR/Cas9 genome editing and several successful reports have been published. Issues regarding yield, quality, and biotic and abiotic stresses faced by the horticultural crops could be solved using CRISPR/Cas9.
REFERENCES
Andersson, M., Turesson, H., Nicolia, A., Fält, A.S., Samuelsson, M. and Hofvander, P. 2017. Efficient targeted multiallelic mutagenesis in tetraploid potato (Solanum tuberosum) by transient CRISPR-Cas9 expression in protoplasts. Plant Cell Reports, 36: 117-128. [Abstract/FREE full text, Google Scholar]
Ashraf, M. 2010. Inducing drought tolerance in plants: recent advances. Biotechnology Advances, 28: 169-183. [Abstract/FREE full text, Google Scholar]
Carroll, D. 2011. Genome engineering with zinc-finger nucleases. Genetics, 188: 773-782. [Abstract/FREE full text, Google Scholar]
Chandrasekaran, J., Brumin, M., Wolf, D., Leibman, D., Klap, C., Pearlsman, M., Sherman, A., Arazi, T. and Gal-on, A. 2016. Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Molecular Plant Pathology, 17: 1140-1153. [Abstract/FREE full text, Google Scholar]
Charrier, A., Vergne, E., Dousset, N.J.-P., Richer, A., Petiteau, A. and Chevreau, E. 2019. Efficient targeted mutagenesis in apple and first-time edition of pear using the CRISPR-Cas9 system. Frontiers in Plant Science, 10: 40. [Abstract/FREE full text, Google Scholar]
Cong, L., Ran, F.A., Cox, D., Lin, S., Barretto, R. Habib, N., Hsu, P.D., Wu, X., Jiang, W., Marraffini, L.A. and Zhang, F. 2013. Multiplex genome engineering using CRISPR/Cas systems. Science, 339: 819-823. [Abstract/FREE full text, Google Scholar]
Corte, L.E., Mahmoud, L.M., Moraes, T.S., Mou, Z., Grosser, J.W. and Dutt, M. 2019. Development of improved fruit, vegetable, and ornamental crops using the CRISPR/Cas9 genome editing technique. Plants, 8: 601. [Abstract/FREE full text, Google Scholar]
Deltcheva, E., Chylinski, K., Sharma, C.M., Gonzales, K., Chao, Y., Pirzada, Z.A., Eckert, M.R., Vogel, J. and Charpentier, E. 2011. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature, 471: 602-607. [Abstract/FREE full text, Google Scholar]
Dutt, M., Barthe, G., Irey, M. and Grosser, J. 2015. Transgenic Citrus Expressing an Arabidopsis NPR1 Gene Exhibit Enhanced Resistance against Huanglongbing (HLB; Citrus Greening). PLoS ONE 10(9): e0137134. [Abstract/FREE full text, Google Scholar]
Jia, H., Zhang, Y., Orbovic, V., Xu, J., White, F.F., Jones, J.B. and Wang, N. 2017. Genome editing of the disease susceptibility gene CsLOB1 in citrus confers resistance to citrus canker. Journal of Plant Biotechnology, 15: 817-823. [Abstract/FREE full text, Google Scholar]
Jiang. F. and Doudna, J.A. 2017. CRISPR–Cas9 structures and mechanisms. Annual Review of Biophysics, 46(1): 505-529. [Abstract/FREE full text, Google Scholar]
Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J.A. and Charpentier, E. 2012. A Programmable dual-RNA – guided DNA endonuclease in adaptive bacterial immunity. Science, 337: 816-822. [Abstract/FREE full text, Google Scholar]
Karkute, S.G., Singh A.K., Gupta, O.P., Singh, P.M. and Singh B. 2017. CRISPR/Cas9 mediated genome engineering for improvement of horticultural crops. Frontiers in Plant Science, 8: 1635. [Abstract/FREE full text, Google Scholar]
Kishi-Kaboshi, M., Aida, R. and Sasaki, K. 2017. Generation of gene-edited Chrysanthemum morifolium using multicopy transgenes as targets and markers. Plant and Cell Physiology, 58: 216-226. [Abstract/FREE full text, Google Scholar]
Klap, C., Yeshayahou, E., Bolger, A., Arazi, T., Gupta, S., Shabtai, S., Usadel B., Salts, Y. and Barg, R. 2017. Tomato facultative parthenocarpy results from SlAGAMOUS-LIKE 6 loss of function. Journal of Plant Biotechnology, 15: 634-647. [Abstract/FREE full text, Google Scholar]
Li, B., Cui, G., Shen, G., Zhan, Z., Huang, L., Chen, J. and Qi, X. 2017. Targeted mutagenesis in the medicinal plant Salvia miltiorrhiza. Scientific Reports, 7: 43320. [Abstract/FREE full text, Google Scholar]
Li, T., Liu, B., Spalding, M.H., Weeks, D.P. and Yang, B. 2012. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nature Biotechnology, 30: 390-392. [Abstract/FREE full text, Google Scholar]
Mahfouz, M.M., Li, L., Shamimuzzaman, M., Wibowo, A., Fang, X. and Zhu, J.K. 2011. De novo-engineered transcription activator-like effector (TALE) hybrid nuclease with novel DNA binding specificity creates double-strand breaks. Proceedings of the National Academy of Sciences of United States of America, 108(6): 2623-2628. [Abstract/FREE full text, Google Scholar]
Makarova, K.S., Grishin, N.V., Shabalina, S.A., Wolf, Y.I. and Koonin, E.V. 2011. A putative RNA-interference-based immune system in prokaryotes: computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action. Biology Direct, 1: 7. [Abstract/FREE full text, Google Scholar]
Mazier, M., Flamain, F., Nicolai, M., Sarnette, V. and Caranta, C. 2011. Knockdown of both eIF4E1 and eIF4E2 genes confers broad-spectrum resistance against Spoty viruses in tomato. Public Library of Science, 6: e29595. [Abstract/FREE full text, Google Scholar]
Nakajima, I., Ban, Y., Azuma, A., Onoue, N., Moriguchi, T., Yamamoto, T., Toki, S. and Endo, M. 2017. CRISPR/Cas9-mediated targeted mutagenesis in grape. Public Library of Science, 12: e0177966. [Abstract/FREE full text, Google Scholar]
Nekrasov, V., Wang, C., Win, J., Lanz, C., Weigel, D., and Kamoun, S. 2017. Rapid generation of a transgene free powdery mildew resistant tomato by genome deletion. Scientific Reports, 7: 482. [Abstract/FREE full text, Google Scholar]
Peng, A., Chen, S., Lei, T., Xu, L., He, Y., Wu, L., Yao, L. and Zou, X. 2017. Engineering canker resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Journal of Plant Biotechnology, 15(12): 1509-1519 [Abstract/FREE full text, Google Scholar]
Rodríguez-Hernández, A.M., Gosalvez, B., Sempere, R.N., Burgos, L., Aranda, M.A. and Truniger, V. 2012. Melon RNA interference (RNAi) lines 29 silenced for Cm-eIF4E show broad virus resistance. Molecular Plant Pathology, 13: 755-763. [Abstract/FREE full text, Google Scholar]
Slavin, J.L. and Lloyd, B. 2012. Health benefits of fruits and vegetables. Advances in Nutrition, 3: 506-516. [Abstract/FREE full text, Google Scholar]
Tester, M. and Langridge, P. 2010. Breeding technologies to increase crop production in a changing world. Science, 327: 818-822. [Abstract/FREE full text, Google Scholar]
Tian, S., Jiang, L., Gao, Q., Zhang, J., Zong, M., Zhang, H., Ren, Y., Guo, S., Gong, G., Liu, F. and Xu, Y. 2017. Efficient CRISPR/Cas9-based gene knockout in watermelon. Plant Cell Reports, 36: 399-406. [Google Scholar]
Wang, L., Wang, L., Tan, Q., Fan, Q., Zhu, H., Hong, Z., Zhang, Z. and Duanmu, D. 2016. Efficient inactivation of symbiotic nitrogen fixation related genes in Lotus japonicas using CRISPR-Cas9. Frontiers in Plant Science, 7: 1333. [Abstract/FREE full text, Google Scholar]
Clustered regularly interspaced short palindromic repeat, Crop improvement, disease resistance, produce quality.
* Corresponding author
a Department of Plant Pathology, University of Agriculture, 38040 Faisalabad, Pakistan
b Department of Horticulture, College of Agriculture, University of Sargodha, 40100 Sargodha, Pakistan
c Centre of Agricultural Biochemistry and Biotechnology, University of Agriculture, 38040 Faisalabad, Pakistan
Email: amjad.ali@uaf.edu.pk (M.A. Ali)
This article does not contain any abbreviations to display here.
Received: 28 March 2020
Revised: 29 June 2020
Accepted: 13 July 2020
Published: 30 September 2020
How to Cite
| AMA |
Zahoor A, Shahzadi S, Niaz Z, et al. CRISPR Cas-9: A genome editing tool for the improvement of horticultural crops: A review. J Hortic Sci Technol. 2020;3(3):59-62. doi:https://doi.org/10.46653/jhst20030359
Polycystic Ovary Syndrome- cysts in ovaries are removed. viagra 50 mg This happens when the man is facing certain disorders which would http://www.donssite.com/steertech/peterbilt-exhaust-steering-repair.htm on line viagra be mentioned below. The medicine works to stop the obstructing action of the enzyme and promoting strong and hard erection.Product is for both men and women:This product is beneficial for no prescription viagra either sex. Tadalafil will be the generic title of viagra soft tab while vardenafil may be the generic title of levitra while vardenafil may be the generic title of levitra prescription while vardenafil may be the generic version of the brand name donssite.com.There was a time when such sexual enhancing medicines were out of reach from men and as a result no proper remedy was found to cure it. |
| MLA |
Zahoor, Adil, et al. “CRISPR Cas-9: A Genome Editing Tool for the Improvement of Horticultural Crops: A Review.” Journal of Horticultural Science & Technology, vol. 3, no. 3, 1, 2020, pp. 59–62, doi:https://doi.org/10.46653/jhst20030359.
|
| APA |
Zahoor, A., Shahzadi, S., Niaz, Z., Shahzadi, M., Jabran, M., Haseeb, A., Anwar, H., Nawaz, M. A., & Ali, M. A. (2020). CRISPR Cas-9: A genome editing tool for the improvement of horticultural crops: A review. Journal of Horticultural Science & Technology, 3(3), 59–62. https://doi.org/10.46653/jhst20030359
|
Download Citation (RIGHT CLICK & “SAVE LINK AS”)
This article do not contain any supplementary data.