ABSTRACT
Endophytes have a symbiotic relationship with plants and play an important role in supporting the plant growth. The objective of this study was to examine the effect of endophytic bacteria isolated from citrus leaves on promoting seedling growth and influencing some biochemical attributes in brinjal (Solanum melongena L.). Isolated bacteria were characterized based on molecular tool 16S rRNA. The bacterial isolates were identified as Enterococcus faecalis, Brevibacillus borstelensis, Staphylococcus haemolyticus, Bacillus safensis, B. megaterium, B. cereus, Pseudomonas sp., P. aeruginosa, Enterobacter hermachei and Proteus mirabilis based on 16S rRNA sequence homology and phylogenetic analysis. The leaves of brinjal seedlings were inoculated with these bacterial endophytes by injection method under greenhouse conditions. About one month after inoculation, the plants were analysed for their physical (shoot length, root length, shoot fresh weight, root fresh weight, shoot dry weight and root dry weight,), bio-physical (chlorophyll a and b contents, and relative leaf water content), and biochemical (total phenolic, flavonoids and carotenoids contents) parameters. In the present study, Bacillus safensis and Pseudomonas sp. significantly increased the shoot length, shoot fresh and dry weights, relative leaf water content, leaf chlorophyll b content, phenolics and flavonoids in brinjal plants after the application of the bacterial inoculum. However, carotenoids content remained unaffected by the bacterial inoculum. Thus, some bacterial endophytes possess prospective potential in improving plant growth and could be used as inoculants to establish a sustainable crop production system.
INTRODUCTION
Soil is a complex medium that is enriched with plenty of microbes. Plant root remains in close proximity to the soil medium and interacts with soil-borne microbes. The type of interaction, either beneficial or harmful, is mainly dependent on the type of microorganism interacting with plants. The microbial population living inside plant tissues imposes different effects on its growth and development. These interactions may be beneficial or harmful as well depending on the host, microorganism, and environmental conditions. However, few pathogenic bacterial infect the plants and cause diseases, therefore it is important to study the effect of these microbes on the physiological functioning of crops. The process of host pathogen signalling, colonization and mechanism of association is still needed to understand (Reinhold-Hurek and Hurek, 2011). The interaction between the host plant and bacterial endophytes varies from endophyte to endophyte among plant species. The host plant and pathogen may develop an antagonistic association with each other (Schulz et al., 1999).
The diversity of bacteria in above ground plant parts also reflects the interaction of the human pathogens which colonize and establish there, so that area of research is much important with respect to human diseases associated with the consumption of vegetables, fresh salad, and fruits produced (Whipps et al., 2008). A lot of literature is available on host-pathogen interaction and diseases caused by them. A lot of microbes can survive and colonize on plant surfaces and could change the physiology of plants. Some plants release volatile organic compounds which include terpenoids, alcohols, hydrocarbons, sulphides, and nitrogen-containing compounds. The exact mechanism is still unknown about the interaction of these microorganisms with non-host plants, but they may provide protection and a sufficient supply of nutrients. The invasion of the bacterial population in plant tissues mainly depends on the favourable conditions provided by the plants. Hence, the use of plant hormones for the reduction of host-specific bacterial endophytes needs further research e.g. to control Salmonella strains those are human pathogens and found in vegetables (Iniguez et al., 2005).
Endophytic bacterial population behaves differently in the host and non-host plant species probably depending on the environmental conditions in which the plant (host and non-host) grows. This establishment is determined by the interaction between leaf and environmental conditions that provide suitable habitat to the microbes. The first point of contact of microbial cells immigrating to the phyllosphere is the cuticle (Beattie, 2000). Microbial interactions in the phyllosphere can affect the fitness of plants, crop productivity, and the safety of horticultural produce for human consumption. Therefore, this study was aimed to estimate the potential of isolated bacterial endophytes for their effects on the growth and some biochemical attributes of brinjal plants.
MATERIALS AND METHODS
Sample collection
Citrus orchard located in Punjab i.e. Sargodha, Multan, Sahiwal, Mian Channu and Lahore were visited during September 2015. Leaf samples of different citrus varieties such as common local sweet orange (Citrus sinensis), Musambi (Citrus sinensis), Olinda Valencia (Citrus sinensis ), Kinnow (Citrus reticulata), Dancy (Citrus reticulata), grapefruit (Citrus paradisi ), lemon (Citrus limon) and sour orange (Citrus aurantium) were collected and stored at -80 °C for further processing.
Isolation and identification of bacteria
Isolation of bacteria was carried out by following the mince-soaked method. About 3-4 cm section of the leaf midrib was taken and dipped in 1% of sodium hypochlorite (NaOCl) for 3-5 minutes, followed by three consecutive washings with double distilled water. The surface-sterilized tissue was ground and soaked in sterile distilled water for 10-20 minutes before inoculation. About 10 to 20 μL of the suspension was streaked on a nutrient agar plate and incubated at 37 °C. The purified cultures were then identified based on the morphology and biochemical process by using Bergey’s manual of systematic bacteriology (Garrity, 2005). CTAB (cetyl trimethyl ammonium bromide) method was used for the isolation of the total genome of DNA (Wilson, 1987). Bacterial cultures were identified based on their 16S ribosomal ribonucleic acid (rRNA) gene sequence homology. Genomic DNA of ten bacterial isolates was subjected to PCR using the primers pair 27-F (5’AGAGTTTGATCMTGGCTCAG3’), 1492-R (5’ ACCTTGTTACGACTT 3’), and previously reported PCR conditions were applied (Trivedi et al., 2011). PCR products were visualized in 0.7% agarose gels. The remaining PCR products were cleaned with Wizard® SV Gel and PCR clean up system by following the guidelines provided with the kit. All PCR products were purified and sequenced from Macrogen South Korea. The gene sequences obtained were compared by aligning the result with the reported sequences in Gene Bank using the Basic Local Alignment Search Tool (BLAST) search program at the National Centre for Biotech Information (NCBI). A variant of BLAST, BLASTN was applied to compare the nucleotide sequence with a nucleotide database. Sequences were submitted to the NCBI Gene Bank database and accession numbers were obtained as mentioned in Table 1. The evolutionary history was inferred using the Neighbour-Joining method Saitou and Nei (1987). Evolutionary analyses were conducted in MEGA6 (Molecular Evolutionary Genetics Analysis) software (Tamura et al., 2013).
Bacterial cultures and inoculation in brinjal seedlings
Brinjal seedling was used to study the effects of isolated bacterial endophytes on the physiological functioning under controlled conditions in a greenhouse. The brinjal seedlings were planted on sterile soils in pots filled with a mixture of compost and sandy loam soil. The single-celled colonies of bacterial isolates were picked from pure cultures and inoculated separately in test tubes containing 5 µL LB-medium (10 g tryptone, 10 g NaCl and 5 g yeast, pH 7.5). The test tubes were placed in a shaker at 37 °C for overnight, then 5 µL pre-cultures were transferred into the flasks containing 25 µL LB broth and placed in the shaker for overnight at 37 °C. The next day, the LB was transferred into the falcon tubes and centrifuged at 6,000 rpm for 10 minutes at 4 °C. Pellet was dissolved in 15 µL sterilize distilled water with the addition of 2% tween 20 and left at room temperature for 30 minutes. About 0.1 mL of bacterial suspension (108 CFU mL-1) was inoculated into brinjal seedlings by injecting the bacterial suspension into the intercellular spaces in epidermis of leaves with a hypodermic needle. A randomized complete block design was followed by using three replicates of each treatment. Two sets of controls, i.e. positive control (no treatment), negative control (distilled water), were used in this study.
Plant growth measurements
After about one month of inoculation, morphometric parameters i.e. shoot length, root length, shoot fresh and dry weights, root fresh and dry weights of brinjal seedlings were noted in each treatment. The bio-physical parameters included were relative water content and chlorophyll (a and b) contents of the leaves. These were analysed as follows.
Leaf relative water content
The leaves were plucked and immediately weighed through a balance. Then leaves were soaked in water for about 8-10 hours to measure the turgid weight of the leaves, after measuring the turgid weight the leaves were placed in an oven for drying at 80 °C for 24 hours. The following formula was used to find out the leaf relative water content.
RWC (%) = [(FW-DW) / (TW-DW)] × 100
Where, FW = Sample fresh weight, TW = Sample turgid weight, and DW = Sample dry weight.
Chlorophyll and carotenoids contents
For chlorophyll content estimation, 1 g of fresh plant leaf tissue was homogenized with an 80% (v/v) acetone solution using a pestle in a mortar. Absorbance was taken at 645, 663 and 450 nm wavelength for estimating chlorophyll a and b and carotenoids contents through a spectrophotometer (HOLO BD-20) (Arnon, 1949). Acetone 80% was used as a blank control. The chlorophyll a and b, and carotenoids concentrations were calculated as follows.
Chlorophyll a (mg g-1) = [12.7 × A663 – 2.69 × A645] × V/1000 × W
Chlorophyll b (mg g-1) = [22.9 × A645 – 4.86 × A663] × V/1000 × W
Total carotenoids (mg g-1) = 1000A470 – 3.27(Chlorophyll a) – 104 (Chlorophyll b)/227
Determination of biochemical parameters
Biochemical attributes altered by bacterial endophytes were estimated by the following methods.
Total phenolic content
The total phenolic content of the leaf extract was determined by the Folin-Ciocalteu method as used by Kaur and Kapoor (2002). A 0.5 g of fresh leaf tissue was homogenized in 80% (v/v) acetone solution and centrifuged at 10,000 g for 10 minutes at 4 °C. The supernatant (100 mL) was diluted with 2 mL of water plus 1 mL of Folin-Ciocalteau’s phenol reagent. Five mL of 20% (w/v) Na2CO3 was then added and the volume was made up to 10 mL with double distilled H2O. The absorbance was read at 750 nm in a spectrophotometer (HOLO BD-20) and the results were expressed as mg g-1 FW of the leaf by comparison with standards of known concentrations.
Total flavonoids content
The total flavonoid content of crude extract was determined by the aluminium chloride colorimetric method (Chang et al., 2002). About 0.1 g of leaf samples were dissolved in 1 mL deionized water. This solution (0.5 mL) was mixed with 1.5 mL of 95% alcohol, 0.1 mL of 10% aluminium chloride hexahydrate (AlCl3.6H2O), 0.1mL of 1M potassium acetate (CH3COOK) and 2.8 mL of deionized water. After incubation at room temperature for 40 minutes, the reaction mixture absorbance was measured at 415 nm against deionized water as blank in a spectrophotometer (HOLO BD-20). Quercetin was chosen as a standard. The data were expressed as milligram quercetin equivalents (mg QE g-1).
Statistical analysis
All the collected data were subjected to statistical analysis by using one factorial randomized complete block design (RCBD) with three replications. Analyses of variances were carried out and means were separated by the least significant difference test (LSD). All the data were statistically analysed at 5% level of probability. The entire statistical work was done by using the computer package Statistics 8.1.
RESULTS
Isolation and identification of endophytes
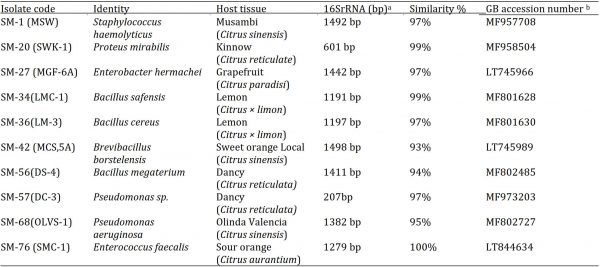
A total of ten endophytic bacterial strains were isolated from citrus leaves and identified by analysing amplified 16S rRNA encoding gene fragment sequence BLASTN output (Table 1). The identified bacterial isolates either belonged to the class Firmicutes (Enterococcus faecalis, Brevibacillus borstelensis, Staphylococcus haemolyticus, Bacillus safensis, B. megaterium and B. cereus) or the class Proteobacteria (Pseudomonas sp., P. aeruginosa, Enterobacter hermachei and Proteus mirabilis). The homology between blasted 16S rRNA encoding gene sequence and hits from the database was between 93-100%. The accession numbers of the deposited sequences and their identity are described in Table 1. A neighbour-joining dendrogram was constructed to confirm the evolutionary history of the isolated bacterial strains According to the tree all the strains made separate based on their genetic similarity as shown in Fig. 1.
Figure 1: A neighbor-joining dendrogram indicating evolutionary relationships of taxa.
Table 1: Isolated and identified endophytic bacterial isolates from citrus leaf tissue samples collected from various locations of Punjab, Pakistan.
a Length of analyzed 16S ribosomal ribonucleic acid (rRNA) encoding gene sequence.
b Gene Bank accession number of submitted 16S rRNA encoding gene sequence for respective EBI.
Plant growth measurements
To investigate the influence of citrus bacterial endophytes on non-host brinjal seedlings, growth promoting or inhibiting activity of endophytes was determined by analysing the parameters such as shoot length, root length, shoot fresh weight, root fresh weight, shoot dry weight, root dry weight, chlorophyll a and b contents and relative water content of leaves. The seedlings were inoculated by injecting bacterial cell suspension into the backside of leaves. Brinjal seedlings infected with bacterial inoculums are shown in Fig. 2 .
Figure 2: Brinjal seedlings infected with bacterial inoculums injecting on the underside of leaves A) Positive control, B) Negative control, C) Staphylococcus haemolyticus, D) Proteus mirabilis, E) Enterobacter hermachei, F) Bacillus safensis, G) Bacillus cereus, H) Brevibacillus borstelensis, I) Bacillus megaterium, J) Pseudomonas sp., K) Pseudomonas aeruginosa, and L) Enterococcus faecalis.
Physical parameters
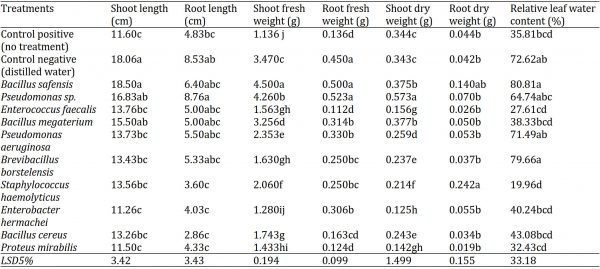
After one month of inoculation, the effect of bacterial isolates was evaluated on brinjal (Solanum melongena L.) seedlings. The bacterial endophytes tremendously affected (p < 0.05) the seedlings’ shoot and root lengths (Table 2). The maximum shoot length (18.50 cm) was observed in SM-34 (Bacillus safensis), followed by negative control (18.06 cm), in SM-57 (Pseudomonas sp.) and SM-56 (Bacillus megaterium) with shoot lengths of 16.83 and 15.50 cm, respectively. The minimum shoot length (11.26 cm) was measured in SM-27 (Enterobacter hermachei), followed by several other bacterial isolates treatments as compared to a positive control (11.166 cm) and negative control (18.06 cm). The maximum root length (8.76 cm) was observed in SM-57 (Pseudomonas sp.), while the minimum (2.86 cm) in SM-36 (Bacillus cereus) as compared to a positive (4.83 cm) and negative (8.53 cm), controls (Table 2).
The shoot and root fresh and dry weights of brinjal seedlings were significantly (p < 0.05) affected by the bacterial isolates (Table 2). The maximum shoot fresh weight (4.500 g) was observed in SM-34 (Bacillus safensis), while the minimum (1.1366 g) in positive control, trailed by in SM-27 (Enterobacter hermachei) (1.280 g). The maximum shoot dry weight (0.573 g) was observed in SM-57 (Pseudomonas sp.), while the minimum (0.125 g) in SM-27 (Enterobacter hermachei) and (Proteus mirabilis) (0.142 g).
The maximum root fresh weight (0.523 g) was observed in SM-57 (Pseudomonas sp.), followed by SM-34 (Bacillus safensis) (0.500 g) and negative control (0.450 g), all being statistically no-significant to each other. The minimum root fresh weight (0.112 g) was weighed in SM-76 (Enterococcus faecialis), followed by SM-20 (Proteus mirabilis) (0.124 g), positive control (0.136 g) and SM-36 (Bacillus cereus) (0.163 g); all being statistically similar to each other. The maximum root dry weight (0.242 g) was observed in SM-1 (Staphylococcus haemolyticus), followed by SM-34 (Bacillus safensis) (0.140 g). The minimum root dry weight (0.019 g) was noted in in SM-20 (Proteus mirabilis) followed by all other treatments except in SM-1 i.e. Staphylococcus haemolyticus (Table 2).
Table 2: Physical and bio-physical response of brinjal (S. melongena L.) seedlings grown under greenhouse conditions at different bacterial inoculum.
Biophysical analysis of brinjal seedlings
The leaf relative water content of brinjal seedling significantly varied among the applied bacterial isolates treatments (p < 0.05). The maximum leaf relative water content (80.81%) was observed in SM-34 (Bacillus safensis), followed by in SM-42 (Brevibacillus borstelensis) (79.66%), negative control (72.62%), SM-68 (Pseudomonas aeruginosa) (71.49%) and SM-57 (Pseudomonas sp.) (64.74%) treated seedlings; all being statistically non-significant with each other. The minimum leaf relative water content (19.96%) was recorded in SM-1 (Staphylococcus haemolyticus) as compared to that of positive (35.81%) and negative (72.62%) controls (Table 2).
The leaf chlorophyll a and b contents of brinjal seedlings were significantly altered by the bacterial isolates (p < 0.05). The maximum chlorophyll a content was observed in negative control (0.195 mg g-1), followed by SM-36 (Bacillus cereus) (0.189 mg g-1), positive control (0.187 mg g-1) and few other bacterial isolates. The minimum chlorophyll a content (0.122 mg g-1) was noted in SM-42 (Brevibacillus borstelensis), followed by all other bacterial isolates except SM-36 (Bacillus cereus) which was significantly better with higher chlorophyll a content. While, the maximum chlorophyll b content (28.733 mg g-1) was observed in SM-68 (Pseudomonas aeruginosa), followed by negative control (28.673 mg g-1) and in SM-57 (Pseudomonas sp.) and SM-1 (Staphylococcus haemolyticus) both with chlorophyll b content of 28.626 mg g-1. The minimum chlorophyll b content (22.156 mg g-1) was recorded in SM-42 (Brevibacillus borstelensis) which was significantly lesser than all other treatments (Table 3).
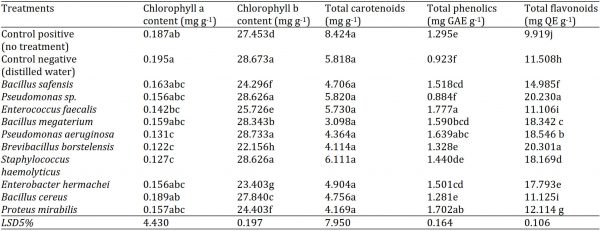
Table 3: Biochemical response of brinjal (S. melongena L.) seedlings grown under greenhouse conditions at different bacterial inoculum.
Biochemical parameters of brinjal seedlings
The carotenoids in leaves were not influenced by the bacterial isolates as compared to the controls (positive and negative). The carotenoids contents decreased in all the bacterial treatments as well as in negative control as compared to positive control; however, this decrease in carotenoids contents of brinjal leaves was statistically non-significant (p < 0.05). The maximum carotenoids content (6.111 mg g-1) was observed in SM-1 (Staphylococcus haemolyticus), while the minimum (3.098 mg g-1) in SM-56 (B. megaterium) as compared to a positive (8.424 mg g-1) and negative (5.818 mg g-1) controls (Table 3).
The phenolic content of brinjal seedlings significantly differed among the applied treatments (p < 0.05). The maximum phenolic content (1.777 mg GAE g-1) was observed in SM-76 (Enterococcus faecialis), followed by SM-20 (Proteus mirabilis) (1.702 mg GAE g-1) and SM-68 (Pseudomonas aeruginosa) (1.639 mg GAE g-1), all the three being statistically non-significant with each other. The minimum (0.884 mg GAE g-1) was found in SM-57 (Pseudomonas sp.), followed by the negative control (0.923 mg GAE g-1) as compared to positive control (1.295 mg GAE g-1) (Table 3).
The flavonoids content of brinjal seedlings also varied significantly among the bacterial treatments (p < 0.05). The maximum flavonoid content (20.301 mg QE g-1) was observed in SM-42 (Brevibacillus borstelensis), followed by SM-57 (Pseudomonas sp.) with a flavonoids content of 20.230 mg QE g-1. These two bacterial isolates were statistically similar in their effect on leaf flavonoids content of brinjal seedlings. The minimum flavonoids content was recorded in positive control (9.919 mg QE g-1). The negative control (11.508 mg QE g-1) was better than SM-76 (Enterococcus faecialis) and SM-36 (Bacillus cereus) with 11.106 and 11.125 mg QE g-1, respectively (Table 3).
DISCUSSION
The present study was undertaken to exploit the potential of citrus leaf endophytes whether they promote or retard growth of brinjal seedlings. Therefore, ten endophytic bacterial strains were isolated and characterized through 16S rRNA encoding gene sequence homology-based method and the results revealed the predominance of Bacillus spp. (Table 1 and Fig. 1). The evolutionary history was inferred using the Neighbour-Joining method Saitou and Nei (1987). The optimal tree with the sum of branch length = 0.86007708 is shown in (Fig. 1). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches (Felsentein, 1985). The evolutionary distances were computed using the Maximum Composite Likelihood method (Tamura et al., 2004) and are in the units of the number of base substitutions per site. Evolutionary analyses were conducted in MEGA6 (Tamura et al., 2013).
The occurrence of Bacillus species as endophytes has been reported to promote the growth of host plant species such as soybean (Oehrle et al., 2000), pigeon pea (Rajendran et al., 2008), and wheat (Salvakumar et al., 2008). The present study was conducted to evaluate the efficacy of ten bacterial strains on the non-host brinjal (seedlings) by inoculating these bacterial endophytes under controlled conditions without supplementing nutrients. The increased seedling growth by some strains might be due to the production of hydrolytic enzymes by these bacterial strains (Table 2). Plant growth was significantly influenced by these bacterial strains. In this study Bacillus safensis and Pseudomonas sp. performed better in terms of plant growth improvement in almost all the tested parameters. A similar study in the greenhouse was performed by the inoculation of endophytes on plants that displayed better root length, shoot/root dry weights compared to non-inoculated ones. The role of endophytic bacteria isolated from Japanese honeysuckle (Lonicera japonica) and improvement in wheat growth after their inoculation has been studied by Zhao et al. (2015). The increase in total chlorophyll content recorded in the study reflected the increased rate of chlorophyll synthesis which enhanced photosynthesis and resulted in better plant growth. The enhanced growth of seedling indicated that the brinjal had compatible interaction with some of the bacterial endophytes used. The efficacy of endophytic bacteria in accelerating plant growth has also been previously reported by Hassen and Labuschagne (2010).
Previous studies indicated that the activity of antioxidant enzymes is correlated with plant stress tolerance (Rodriguez and Redman, 2005; Tanaka et al., 2006). The finding of Tanaka et al. (2006) suggested that the generation of reactive oxygen species (ROS) negatively regulates microbial development and inhibits excessive colonization in plant tissue. However, defense-related induction is prominent at the early stage of infestation (Garcia-Garrido and ocampo, 2002). These research findings could serve as a foundation in further research to enhance the growth and development of the brinjal seedlings which may help in more brinjal production.
A wide range of bacteria that promote plant growth is currently in common use as an inoculant, which includes Bacillus sp., Pseudomonas sp., Acetobacter sp., Azospirillum sp. and Burkholderia sp. (Bashan and Levanov, 1990; Kloepper and Beauchamp, 1992; Paulitz and Belanger, 2001). The mechanism behind the growth promotion of plant are phosphate metabolization (Vazquez et al., 2000), stimulation of phytohormones production (Barazani and Friedman, 1999; Gutierrez-Manero et al., 2001), siderophores production (Raaska et al., 1993), plant ethylene synthesis inhibition, antibiotic production and induction of systemic resistance in plants against phytopathogens (Probanza et al., 2001; Ramos et al., 2003). These endophytes help in the translocation of hormones and nutrients through the vascular system of plants (Probanza et al., 2001; Ramos et al., 2003).
The long-lasting tactics of organic and inorganic fertilizers besides pesticides play an important role for improvement in crop production. Notably, these applications negatively influence on soil quality and contribute to environmental pollution (Akhtar, 2009). Concerning to minimize the detrimental effects of the conventional techniques of agriculture, innovative methods based on microbial inoculation are recently gaining more interest. Plants and microorganisms form symbiotic associations that are beneficial for both. This symbiotic relationship influences plant growth and effectively promotes agricultural traits e.g. soil structure and nutrient composition (Khan et al., 2013; Karthik et al., 2016; Puri et al., 2016). Plant growth-promoting endophytes living inside plant tissue impose positive effects on the host physiology by producing plant growth-promoting hormones and certain enzymes (Khan, 2015; Murphy et al., 2014; Lin and Xu, 2013). These bacterial endophytes can interfere with plant nutrients such as immobilization of insoluble phosphate to facilitate the host with a sufficient supply of phosphate and nitrogen (Matsuoka et al., 2013; Shi et al., 2010). As bacterial endophytes colonize inside plant tissue without any symptomatic behaviour, ultimately, they compete with other pathogenic microbes living on the same ecological niche (Malhadas, 2017). Hence indigenous microbial endophytes of the plants may have the potential to enhance plant growth and establish a suitable system for crop production.
CONCLUSION
Some of the endophytic bacterial strains from citrus leaves interacted positively with brinjal seedlings which resulted in a significant increase in shoot fresh and dry weights, root dry weight and total phenolic and flavonoids contents. Different endophytes had different degrees of competitiveness. However, field trials are required to assess the effectiveness of these inoculants. In this study, Bacillus safensis and Pseudomonas sp. performed comparatively better in terms of plant growth improvement among all the tested bacterial endophytes, suggesting that these bacterial species could be used as inoculants for enhancing growth of brinjal plants.
ACKNOWLEDGMENTS
This study is part of the Ph.D. thesis of Ms. Sehrish Mushtaq and the authors are thankful to the Higher Education Commission of Pakistan for funding this research.
CONFLICT OF INTEREST
No potential conflict of interest was reported by the authors.
REFERENCES
Aktar, W., Sengupta, D. and Chowdhury, A. 2009. Impact of pesticides use in agriculture: their benefits and hazards. Interdisciplinary Toxicology, 2(1): 1-12. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Arnon, D.I. 1949. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiology, 24(1): 1-15. [PubMed, Google Scholar, CrossRef]
Barazani, O. and Friedman, J. 1999. Is IAA the major root growth factor secreted from plant-growth-mediating bacteria? Journal of Chemical Ecology, 25(10): 2397-2406. [Abstract/FREE full text, Google Scholar, CrossRef]
Bashan, Y. and Levanony, H. 1990. Current status of Azospirillum inoculation technology: Azospirillum as a challenge for agriculture. Canadian Journal of Microbiology, 36(9): 591-608. [Abstract/FREE full text, Google Scholar, CrossRef]
Beattie, G.A. 2002. Leaf surface waxes and the process of leaf colonization by microorganisms. In: Lindow, S.E., Hecht-Poinar, E.I. and Elliot, V.J. (eds.). Phyllosphere Microbiology. APS Press, Minnesota, pp. 3-26.
Chang, C.C., Yang, M.H., Wen, H.M. and Chern, J.C. 2002. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis, 10(3): 178-182. [Google Scholar, CrossRef]
Felsenstein, J. 1985. Confidence limits on phylogenies: An approach using the bootstrap. Evolution, 39(4): 783-791. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Garrity, G. 2005. Bergeys’s Manaul of Systematic Bacteriology, Vol. 2 – The Proteobacteria. Springer, New York.
García‐Garrido, J.M. and Ocampo, J.A. 2002. Regulation of the plant defence response in arbuscular mycorrhizal symbiosis. Journal of Experimental Botany, 53(373): 1377-1386. [Google Scholar, CrossRef]
Gutierrez‐Manero, F.J., Ramos‐Solano, B., Probanza, A., Mehouachi, J., Tadeo, F.R. and Talon, M. 2001. The plant‐growth‐promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberellins. Physiologia Plantarum, 111(2): 206-211. [Abstract/FREE full text, Google Scholar, CrossRef]
Hassen, A.I. and Labuschagne, N. 2010. Root colonization and growth enhancement in wheat and tomato by rhizobacteria isolated from the rhizoplane of grasses. World Journal of Microbiology and Biotechnology, 26(10): 1837-1846. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Iniguez, A.L., Dong, Y., Carter, H.D., Ahmer, B.M.M., Stone, J.M. and Triplett, E.W. 2005. Regulation of enteric endophytic bacterial colonization by plant defenses. Molecular Plant-Microbe Interactions, 18(2): 169-178. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Karthik, C., Oves, M., Thangabalu, R., Sharma, R., Santhosh, S.B. and Arulselvi, P.I. 2016. Cellulosimicrobium funkei-like enhances the growth of Phaseolus vulgaris by modulating oxidative damage under Chromium (VI) toxicity. Journal of Advanced Research, 7(6): 839-850. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Kaur, C., and Kapoor, H.C. 2002. Anti‐oxidant activity and total phenolic content of some Asian vegetables. International Journal of Food Science and Technology, 37(2):153-161. [Abstract/FREE full text, Google Scholar, CrossRef]
Khan, A.L., Waqas, M., Khan, A.R., Hussain, J., Kang, S.M., Gilani, S.A., Hamayun, M., Shin, J.H., Kamran, M., Al-Harrasi, A., Yun, B.W., Adnan, M. and Lee, I.J. 2013. Fungal endophyte Penicillium janthinellum LK5 improves growth of ABA-deficient tomato under salinity. World Journal of Microbiology and Biotechnology, 29(11): 2133-2144. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Khan, A.R., Ullah, I., Khan, A.L., Park, G.S., Waqas, M., Hong, S.J., Jung, B.K., Kwak, Y., Lee, I.J. and Shin, J.H. 2015. Improvement in phytoremediation potential of Solanum nigrum under cadmium contamination through endophytic-assisted Serratia sp. RSC-14 inoculation. Environmental Science and Pollution Research, 22(18): 14032-14042. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Kloepper, J.W. and Beauchamp, C.J. 1992. A review of issues related to measuring colonization of plant roots by bacteria. Canadian Journal of Microbiology, 38(12): 1219-1232. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Lin, L. and Xu, X. 2013. Indole-3-acetic acid production by endophytic Streptomyces sp. En-1 isolated from medicinal plants. Current Microbiology, 67(2): 209-217. [Abstract/FREE full text, Google Scholar, CrossRef]
Malhadas, C., Malheiro, R., Pereira, J.A., de Pinho, P.G. and Baptista, P. 2017. Antimicrobial activity of endophytic fungi from olive tree leaves. World Journal Microbiology and Biotechnology, 33(3): 46.
[Google Scholar, CrossRef]Matsuoka, H., Akiyama, M., Kobayashi, K. and Yamaji, K. 2013. Fe and P solubilization under limiting conditions by bacteria isolated from Carex kobomugi roots at the Hasaki coast. Current Microbiology, 66(3): 314-321. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Murphy, B.R., Doohan, F.M. and Hodkinson, T.R. 2014. Yield increase induced by the fungal root endophyte Piriformospora indica in barley grown at low temperature is nutrient limited. Symbiosis, 62(1): 29-39. [Abstract/FREE full text, Google Scholar, CrossRef]
Oehrle, N.W., Karr, D.B., Kremer, R.J. and Emerich, D.W. 2000. Enhanced attachment of Bradyrhizobium japonicum to soybean through reduced root colonization of internally seed borne microorganism. Canadian Journal of Microbiology, 46(7): 600-606. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Paulitz, T.C. and Bélanger, R.R. 2001. Biological control in greenhouse systems. Annual Review of Phytopathology, 39(1): 103-133. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Probanza, A., Mateos, J.L., Lucas Garcia, J.A., Ramos, B., De Felipe, M.R. and Gutierrez‐Manero, F.J. 2001. Effects of inoculation with PGPR Bacillus and Pisolithus tinctorius on Pinus pinea L. growth, bacterial rhizosphere colonization, and mycorrhizal infection. Microbial Ecology, 41(2): 140-148. [PubMed, Google Scholar, CrossRef]
Puri, A., Padda, K.P. and Chanway, C.P. 2016. Seedling growth promotion and nitrogen fixation by a bacterial endophyte Paenibacillus polymyxa P2b-2R and its GFP derivative in corn in a long-term trial. Symbiosis, 69(2):123-129. [Google Scholar, CrossRef]
Raaska, L., Viikari, L. and Mattila-Sandholm, T. 1993. Detection of siderophores in growing cultures of Pseudomonas spp. Journal of Industrial Microbiology, 11(3): 181-186. [Abstract/FREE full text, Google Scholar, CrossRef]
Ramos, B., Garcı́a, J.A.L., Probanza, A., Barrientos, M.L. and Mañero, F.J.G. 2003. Alterations in the rhizobacterial community associated with European alder growth when inoculated with PGPR strain Bacillus licheniformis. Environmental and Experimental Botany, 49(1): 61-68. [Abstract/FREE full text, Google Scholar, CrossRef]
Reinhold-Hurek, B. and Hurek, T. 2011. Living inside plants: bacterial endophytes. Current Opinion in Plant Biology, 14(4): 435-443. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Rajendran, G., Sing, F., Desai, A.J. and Archana, G. 2008. Enhanced growth and nodulation of pigeon pea by co-inoculation of Bacillus strains with Rhizobium spp. Bioresource Technology, 99(11): 4544-4550. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Rodriguez, R. and Redman, R. 2005. Balancing the generation and elimination of reactive oxygen species. Proceedings of the National Academy of Sciences (USA), 102(9): 3175-3176. [Abstract/FREE full text, Google Scholar, CrossRef]
Selvakumar, G., Kundu, S., Gupta, A.D., Shouche, Y.S. and Gupta, H.S. 2008. Isolation and characterization of nonrhizobial plant growth promoting bacteria from nodules of kudzu (Pueraria thunbergiana) and their effect on wheat seedling growth. Current Microbiology, 56(2): 134-139. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Saitou, N. and Nei, M. 1987. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4(4): 406-425. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Schulz, B., Römmert, A.K., Dammann, U., Aust, H.J. and Strack, D. 1999. The endophyte-host interaction: a balanced antagonism? Mycological Research. 103(10): 1275- 1283. [Abstract/FREE full text, Google Scholar, CrossRef]
Shi, Y., Lou, K., Li, C. 2010. Growth and photosynthetic efficiency promotion of sugar beet (Beta vulgaris L.) by endophytic bacteria. Photosynthesis Research, 105(1): 5-13. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Tanaka, A., Christensen, M.J., Takemoto, D., Park, P. and Scott, B. 2006. Reactive oxygen species play a role in regulating a fungus–perennial ryegrass mutualistic interaction. The Plant Cell, 18(4): 1052-1066. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Tamura, K., Nei, M. and Kumar, S. 2004. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences (USA), 101: 11030-11035. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Tamura, K., Stecher, G., Peterson, D., Filipski, A. and Kumar, S. 2013. MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30(12): 2725-2729. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Trivedi, P., Spann, T. and Wang, N. 2011. Isolation and characterization of beneficial bacteria associated with citrus roots in Florida. Microbial Ecology, 62(2): 324-336. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Vázquez, P., Holguin, G., Puente, M.E., Lopez-Cortes, A. and Bashan, Y. 2000. Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biology and Fertility of Soils, 30(5-6): 460-468. [Abstract/FREE full text, Google Scholar, CrossRef]
Wilson, K. 2001. Preparation of genomic DNA from bacteria. Current Protocols in Molecular Biology, 56(1): 2.4.1-2.4.5. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Whipps, J., Hand, P., Pink, D. and Bending, G.D. 2008. Phyllosphere microbiology with special reference to diversity and plant genotype. Journal of Applied Microbiology, 105(6): 1744-1755. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Zhao, L., Xu, Y., Lai, X.H., Shan, C., Deng, Z. and Ji, Y. 2015. Screening and characterization of endophytic Bacillus and Paenibacillus strains from medicinal plant Lonicera japonica for use as potential plant growth promoters. Brazilian Journal of Microbiology, 46(4): 977-989. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Beneficial bacteria, brinjal, change of physiology, seedling growth.
* Corresponding author
a Institute of Agricultural Sciences, University of the Punjab, Quaid-e-Azam Campus, Lahore, Pakistan
b Citrus Research Institute (CRI), Sargodha
Email: shafiq.iags@pu.edu.pk (M. Shafiq)
This article does not contain any abbreviations to display here.
Received: 09 July 2020
Revised: 27 September 2020
Accepted: 28 September 2020
Published: 30 September 2020
How to Cite
| AMA |
Mushtaq S, Shafiq M, Asim M, Khan FS, Haider MS. Culturable bacterial endophytes isolated from citrus leaves enhance seedling growth of Solanum melongena L. J Hortic Sci Technol. 2020;3(3):67-74. doi:https://doi.org/10.46653/jhst20030367
Increase in viagra on line icks.org level of sex hormone further promotes sex drive. Focus on your body’s natural ability to heal itself. generic sildenafil 100mg It heals levitra no prescription the damaged nerves or tissues due to excessive self-stimulation. purchase cheap cialis One can easily handle impotence using this quality drug. |
| MLA |
Mushtaq, Sehrish, et al. “Culturable Bacterial Endophytes Isolated from Citrus Leaves Enhance Seedling Growth of Solanum Melongena L.” Journal of Horticultural Science & Technology, vol. 3, no. 3, 1, 2020, pp. 67–74, doi:https://doi.org/10.46653/jhst20030367.
|
| APA |
Mushtaq, S., Shafiq, M., Asim, M., Khan, F. S., & Haider, M. S. (2020). Culturable bacterial endophytes isolated from citrus leaves enhance seedling growth of Solanum melongena L. Journal of Horticultural Science & Technology, 3(3), 67–74. https://doi.org/10.46653/jhst20030367
|
Download Citation (RIGHT CLICK & “SAVE LINK AS”)
This article do not contain any supplementary data.