Copyright: © 2021 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution 4.0 International License. J. Hortic. Sci. Technol. © 2021 Pakistan Society for Horticultural Science.
ABSTRACT
The tuber dormancy is an important aspect of tuber’s physiological age and begins with tuber initiation. It is largely dependent on genotype, environmental conditions, and tuber age. The group Phureja among diploid potatoes, has a very short or no tuber dormancy while the tubers of Solanum jamesii, a wild potato species, may remain dormant for more than eight years and have the tendency to sprout in favourable conditions. The dormancy breakage in potato is accompanied by many physiological changes such as changes in the ratios of abscisic acid (ABA)/ cytokinin and ABA/ gibberellic acid (GA3), catalase inhibition and accumulation of soluble sugars. These all changes are interlinked and occur in the same time frame. The dormant buds have 77% of their nuclei in the growth phase (G1), compared to only 13% in the preparation phase for mitosis (G2), resulting in slower development of active buds. This paper reviews various factors involved in natural and forced dormancy breakage of potato tuber in relation to their use as seed potatoes immediately after harvesting and implementation of different exogenous dormancy breaking methods like cold pre-treatment, growth regulators, electric current and irradiation to induce sprouting in potatoes.
INTRODUCTION
Potato outlooks as an imperious food crop mainly due to its starch contents, protein, minerals, considerable amounts of essential vitamins, as well as very low amount of fats (King and Slavin, 2013). Nutrients like potassium and phosphorus are abundant in tuber but scarce in the case of sodium and calcium. Iron, magnesium, zinc, manganese, silicon, sulfur, and chlorine are also present in an average amount. There are traces of boron, iodine, bromine, copper, aluminium, molybdenum, cobalt, and nickel (Andre et al., 2007). It positions 4th behind the cereals, rice, wheat, and maize; imparts a fundamental role in food security and world economic development. Its origin is southern America. It is now grown in more than 141 countries (Haase, 2008). The traces reveal potato intentional cultivation date back to 4000 BC. Its tubers, as a general, persist a period of dormancy for about 2-3 months after harvesting. Dormancy is a survival mechanism involving physiological modification to irregular periods of environmental stress (Suttle, 2007). Dormancy and sprouting behaviours of tuber are predicted as major components to study for a promising clone to be released (Virtanen et al., 2013). As a concern to the farming community, it has now become fundamental for them to adapt management strategies for monitoring tuber dormancy; aimed to put their produce on sale at various kinds of food as well as seed industries. This depends undoubtedly on the form of harvest usage. If objective is intended to use tubers as seed, exposure to the temperature above 3 °C and diffused light develop robust sprouts. To exploit the market of potato, there should be the ability to manipulate the natural period of dormancy utilizing appropriate germplasm, chemicals (non-hazardous sprout suppressants and dormancy-inducing), storage, irradiations, and electric current. This dormancy-breaking technology would allow for more flexibility in potato tuber marketing, particularly for export markets, and would also be valuable for plant breeding and disease testing.
POTATO TUBER DORMANCY AND ITS BASIS
Tuber dormancy is a temporary suspension of sprouting from the eyes of the buds containing meristem (Struik and Wiersema, 1999). So, potato tubers due to dormancy do not sprout readily after harvest even if promising ecological conditions are prevailing. The extent of dormancy is essentially considerable based on the use of potato tubers. An extensive period of dormancy is required when the tuber is used for culinary purposes. On the contrary, a similar extension causes trouble in the sprouting of seed tubers for the early crop stand. The tuber dormancy duration is largely dependent on the genotype along with pre-and postharvest conditions. Therefore, the characterization of germplasm under different storage environments is desired to optimize storability that directly or indirectly regulates the keeping quality of potatoes (Carli et al., 2010). Tuber dormancy in potato is divided into three distinguished categories. During its life cycle, potato crops can get through all three kinds of dormancies. “Endodormancy” that results as a consequence of the internal or physiological causes (Suttle, 1998). In this state, tuber sprouting will not occur even if tubers are kept even in favourable conditions (Aksenova et al., 2013). “Ecodormancy” occurs when environmental factors hinder or delay sprouting. For instance, tubers kept at warmer temperatures, possess a shorter dormancy than those stored at lower temperatures. “Paradormancy” is caused by the physiological signal that originates in a different plant part from where the dormancy occurs. Some cultivars have a higher level of paradormancy than others. Endodormancy is terminated when visible sprouting takes place from the apical bud, paradormancy is marked by apical dominance and ecodormancy by the emergence of axillary buds or proximal buds (Bajji et al., 2007).
Ecodormancy is supposed to be regulated by the relative concentrations of the indole acetic acid and gibberellic acid (Sorce et al., 2009). Tuber initiation is the start of dormancy (Sonnewald and Sonnewald, 2014; Awologbi and Hamadina, 2016). Due to the difficulty of measuring this interval, postharvest dormancy is used instead. It is the time between dehaulming and when 80% of tubers have sprouts of at least 2 mm in length (Van Ittersum and Scholte, 1992). During the multiplication of potato and its genetics studies, the short generation is desirable. Numerous techniques at the present are being used worldwide to shorten the tuber and seed dormancy in potato. Many chemicals have been investigated so far to examine their capability of terminating dormancy and induce premature sprouting. Among these chemicals, ethylene chlorhydrin, thiocyanates, bromoethane, and ethylene dichloride showed a strong tendency to terminate dormancy. However, their application raises consumer concerns.
Understanding the basis of short dormancy in diploid potatoes
Cultivated tetraploids produce the highest yields but cultivated diploid potatoes have more potential than is yet appreciated (De Maine, 1996). The tetrasomic inheritance of tetraploids makes potato breeding complicated. On the other hand, selection progress is more rapid with diploids due to their simple disomic inheritance. Increasing favourable gene frequencies at the 4x level is slower than at the 2x level (Jansky, 2009). A population of diploid hybrids S. phureja × S. stenotomum was cultivated in the United States for its ability to adapt to long days, tuber appearance, and high dry matter content (Haynes and Haynes, 1983). This population has many valuable traits: high carotenoid content (Lu et al., 2001) and resistance to several diseases, i.e., early blight (Christ and Haynes, 2001), soft rot Erwinias (Wolters and Collins, 1995) and late blight (Costanzo et al., 2005). The population is characterized by short-dormancy and efforts are underway to lengthen tuber dormancy in the population to make it better adapted to the single cropping season in the US (Haynes, personal communication). However, the genetic variance for tuber dormancy is very high in S. phureja and S. stenotomum populations (Thompson et al., 1980) and the breeding effort to lengthen tuber dormancy is only just beginning, making this population a valuable genetic source for developing short-dormancy potato germplasm.
Bilateral (BSP) or unilateral (USP) sexual polyploidization can be used for the development of diploid clones with desired traits that also produce 2n gametes which can be selected for the development of tetraploid hybrids. BSP involves crosses in which 2n eggs from the diploid female parent and 2n pollen from the diploid male parent unite to form a tetraploid. By contrast, USP is based on crosses between a tetraploid and a diploid, where the diploid parent produces 2n gametes (either 2n pollen or 2n eggs). The use of 2n gametes and the potato’s asexual reproduction mechanism gives breeders a one-of-a-kind opportunity to accumulate non-additive genetic effects at the tetraploid stage.
STRUCTURE OF A POTATO TUBER
It is essential to elaborate the tuber’s structures to understand the physiology of tuber dormancy. Botanically, potato tuber is a modified stem that expands to store nutrients and is a means of vegetative propagation (Longman, 1993). Like other vegetative organs, it overwinters (Okubo, 2000). The tuber has an entire apparatus that a normal stem contains, including nodes, internodes, and axillary buds. The nodes are the eyes, all of which have individual leaf scars (Mauseth, 2012). The nodes/eyes have covered the whole surface of tuber in a spiral fashion starting from the opposite end of stolon attachment ( Cutter, 1992). The development of the terminal bud takes place at the farthest point away from the stolon attachment. Consequently, tubers exhibit the same phenomenon “apical dominance” as in a normal stem (Haverkort et al., 1990). Internally, a tuber is full of starch stored in the swollen parenchyma cells. Inside a tuber, it has typical cell constituents of any stem comprising pith, vascular tissues, and cortex. Cortex is separating the periderm from the vascular ring and pith (Cutter, 1978).
UNDERSTANDING THE PHYSIOLOGY OF TUBER DORMANCY
Sprouting is prompted through many physiological changes including changes in hormonal regulation, antioxidants system and starch metabolism. Abscisic acid (ABA)/ cytokinin and ABA/ gibberellic acid (GA3) balance are regulating factors for bud break and sprout growth progressively. Catalase inhibition, likewise, in tubers, causes oxidative stress and results in breaking tuber dormancy. In case of starch metabolism, reducing and non-reducing sugars start increasing during dormancy transition. Dormancy break is dependent on the availability of sucrose. As a result, sucrose is likely to act as both a nutrient and a signal molecule. However, the mechanism of sucrose sensing is unclear.
ABA has been shown to be essential in controlling tuber dormancy and ABA content in tubers during dormancy is the product of a synthesis-metabolism balance that favours catabolism as dormancy ends (Destefano-Beltran et al., 2006). ABA is formed from the C40 zeaxanthin by several enzymatic steps (Xiong and Zhu, 2003). Zeaxanthin is converted to violaxanthin by zeaxanthin epoxidase (ZEP). The recessive homozygous zep1 allele leads to the accumulation of zeaxanthin at the expense of the downstream carotenoids violaxanthin, antheraxanthin and neoxanthin.
White and yellow flesh traits are under single gene control, [beta-carotene hydroxylase 2 (Chy2)] (Wolters et al., 2010). Only one dominant allele among 11 Chy2 has a major effect, changing white into yellow flesh colour. The more intense yellow-flesh, almost orange, potatoes are particularly high in zeaxanthin (Brown et al., 2006). The presence of dominant Chy2 allele with homozygous recessive Zep1 allele cause accumulation of large amounts of zeaxanthin. The most intense yellow-flesh colours are found in the cultivated diploid potato species S. tuberosum Groups Phureja and Stenotmum (Lu et al., 2001; Brown et al., 2006). Group Phureja, in particular, has very short tuber dormancy, allowing for two cropping seasons a year at its centre of origin in South America (Glendinning, 1983). Haynes and Haynes (1983) adapted a hybrid population of Phureja-Stenotomum to North Carolina, USA. The population maintained its short dormancy during the process of adaptation to NC and may, therefore, serve as a valuable genetic resource for developing cultivars with short dormancy for regions that can produce two crops a year.
MECHANISM OF DORMANCY AT CELLULAR LEVEL
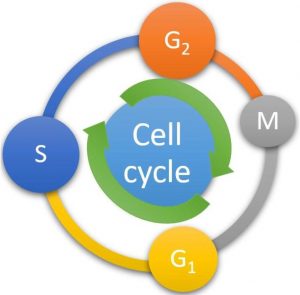
Dormancy is a component of the plant’s genetic design. Tubers have developed this phenomenon to protect against unfavourable conditions. The cell cycle has total four distinct phases, namely growth phase (G1), DNA synthesis phase (S), preparation phase for mitosis (G2) and mitosis phase (M) phase (Fig. 1). Suttle (1996) discovered that 77% of dormant buds’ nuclei are in the G1 phase, with just 13% in the G2 phase. So, it is evident from his findings that growth has not terminated during dormancy, but is ongoing, but at a much slower rate than in an active bud. Between M-phase and S-phase is the G1 phase, and between S-phase and M-phase is the G2 phase (Fairbanks and Anderson, 1999). In addition to these two stages, there is a third stage, G0, in which non-cycling cells are halted. Cells prepare for the next stage of the cell cycle during all G-phases. There are cyclin-dependent kinase (cdk) checkpoints between the G1-S and G2-M stages (Francis and Sorrell, 2001). Suttle (1998) discovered a group of regulatory proteins known as P-34 kinases, the most important of which is cdc2 kinase, which is involved in cell division. Dcyclins regulate these proteins and are needed in plants for the transition from the G1 to the S phase. D-cyclins can react on external signals and bind to Cdk proteins in response to cytokinins, causing the G1 transition to begin (Francis and Sorrell, 2001).
Figure 1: The cell cycle illustrating different phases. G1 = growth phase, S = DNA synthesis phase, G2 = preparation phase for mitosis, and M = mitosis phase.
FACTORS AFFECTING TUBER DORMANCY
Tuber dormancy is regulated by several factors, including genetic background, ecological conditions during tuber growth, extent of tuber maturity at harvest and postharvest circumstances.
Genetic background
Tuber dormancy differs among genotypes (Claassens et al., 2005). Length of potato tuber dormancy is naturally longer in wild genotypes when compared with accessions developed by modern breeding (Suttle, 2007). An exception to this rule is Solanum tuberosum Group Phureja, which produces short dormant tubers. However, tubers of S. jamesii, a wild relative of potato, may remain dormant for more than eight years and still have the potential to sprout in a favourable environment (Bamberg, 2010). Simmonds (1964) revealed the inheritance of tuber dormancy for the first time. He characterized dormancy as a polygenic character and at least three genes are involved in dormancy control. True potato seed (TPS) have a more extended dormancy period than tubers. Since tuber dormancy is not correlated with plant maturity, it is possible to cross early maturing clones with relatively long dormancy and late maturing clones with relatively short dormancy (Beukema and van der Zaag, 1990). Typically, however, early maturing clones have a shorter tuber dormancy that is easier to break than that of late maturing clones.
The wild and cultivated diploids provide breeders with a tremendous germplasm resource (Jansky, 2000). Their genes for traits like tuber dormancy, disease resistance and stress tolerance are easily accessible through modified conventional breeding schemes. Haploids of cultivated tetraploid potato can be derived through parthenogenesis (Hougas et al., 1958). Many diploid relatives readily cross with these haploids, resulting in vigorous interspecific hybrids. Unilateral (4x-2x) or bilateral (2x-2x) sexual polyploidization may be used to make these hybrids tetraploid (Chase, 1963). Quantitative trait loci on chromosomes 2, 3 and 7 have been reported as good candidates for markers assisted association with release of potato tuber dormancy (Bisognin et al., 2018).
Role of environmental conditions
Ecology during tuber growth is a principal factor in shaping tuber dormancy. As tuber dormancy commences during tuber formation, it is presumed that the factors affecting tuberization (e.g., nutrient, water supply, temperature, and day length), also affect dormancy and sprouting (Suttle, 2007). Long-day cultivated potatoes have a longer dormancy period than short-day cultivated potatoes. High temperature adversely affects the tuber formation as it causes tuber chain formation and premature sprouting (Levy and Veilleux, 2007). It is accompanied by low soil moisture and low soil fertility, it hastens maturity and reduces the dormancy period (CIP, 1985).
Physiological tuber age
Tuber dormancy varies with physiological maturity of tubers. This difference can be explained in terms of tuber size, with smaller tubers of the same clone having longer dormancy than larger ones. The onset of sprout growth is influenced by physiological age of the potato tuber. Early-harvested tubers have longer dormancy as compared to later harvested tubers. This difference in dormancy period can be of several weeks.
Postharvest physiological development of tuber
The postharvest development of the tuber towards its ultimate fate is an intricate and sequential process, which primarily involves cell division and cell proliferation to induce visible sprouts (Teper-Bamnolker et al., 2010). Sprouting potential is maximized at full-maturity and declines after a period of storage (Sharma et al., 2020). A tuber undergoes following different phases during its development which may occur simultaneously or sequentially.
Apical dominance: Apical dominance is a physiological phenomenon in which a single sprout develops and dominates over laterals (Pavlista, 2004). Typically, the apical bud is the dominant one.
Multiple sprouting: This stage is characterized by the release of apical dominance and sprouting of several buds. The duration of apical dominance is a genetic characteristic and varies among cultivars (Carli et al., 2010).
Middle age: Seed tubers at this stage exhibit multiple sprouts and are ideal for planting. Removal of apical bud to induce multiple sprouting and sometimes second desprouting would need to be done otherwise apical dominance may reinstate (Carli et al., 2010; Aksenova et al., 2013).
Branching: Branching of tubers occurs shortly after middle age. The branches that arise look “hairy” as they tend to be weak (Leyser, 2009). These developing sprouts use tuber as source of growth. When several sprouts start developing on the tuber, an inter-sprout competition results, which may have little effect on large tubers but a larger effect with decreasing size of tuber. This competition has been shown to be independent of the distance between the competing sprouts, suggesting that it is not a local matter but of growth factors distributed throughout the tuber (Fernie and Willmitzer, 2001).
Weight loss: Weight loss is not a stage as such. Weight loss occurs in storage as the postharvest life progresses. It directly regulates the longevity of tubers, hence their storage quality. Tuber weight loss is predominantly governed by periderm characteristics and sprouting behaviour. In unsprouted tubers, genotypes with a thick periderm and low number of lenticels have comparatively less weight loss than those with a thin periderm and/or a greater number of lenticels (Ezekiel et al., 2004). In sprouted tubers, more weight is lost than in unsprouted ones. A strong correlation exists between weight loss and length and number of sprouts (Pande et al., 2007).
ROLE OF PHYTOHORMONES
Tuber dormancy is presumably controlled by the relative concentrations of growth promoters and inhibitors (Hemberg, 1985). Growth inhibitors i.e., ABA and ethylene are required for dormancy induction but only ABA is needed to maintain the dormancy. GA and cytokinins are assumed to regulate the termination of dormancy (Suttle, 2004a).
Abscisic acid (ABA)
ABA is a naturally occurring plant growth inhibitor. It is produced in chloroplasts and other plastids of the cell (Arteca, 1996). ABA plays a critical role in dormancy regulation. The level of ABA is at the peak in freshly harvested tubers and declines during the postharvest storage period (Suttle, 1996; Claassens and Vreugdenhil, 2000). ABA protects the tuber from cold damage by inhibiting DNA and RNA synthesis and keeping the cell in the G1 phase of the cell cycle until the GA:ABA ratio reaches a certain point which favours gibberellins and hence promotes cell division and sprouting. However, there is no definitive level of ABA below which sprouting commences (Claassens and Vreugdenhil, 2000). Recently NOS-like or NR-generated NO has been found to release potato tuber dormancy and initiate sprouting via ABA metabolism and signalling in tuber buds (Wang et al., 2020).
Cytokinins
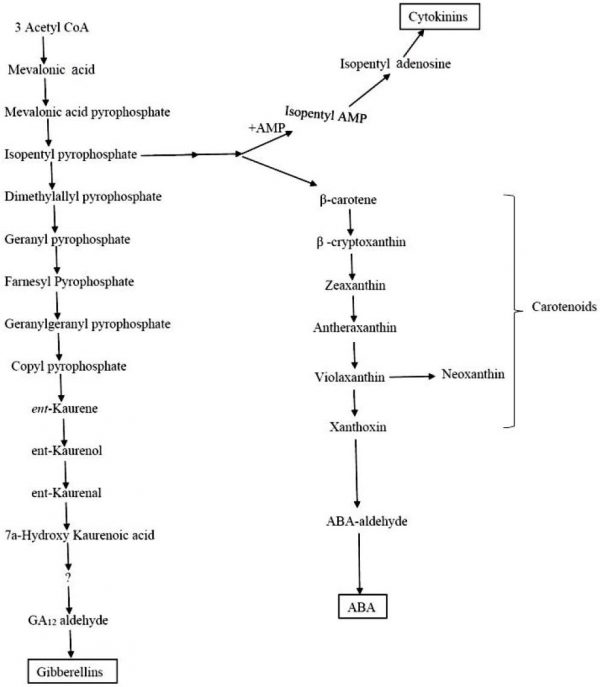
All cytokinins in plants are synthesized from isopentyladenosine, through the mevalonic acid pathway (Vivanco and Flores, 2000) (Fig. 2). The most common cytokinins possess a N6-side chain like zeatin, isopentyladenine and N6-benzyl adenine (Mok and Mok, 2001). Cytokinins affect the cell cycle’s G1-S and G2-M stages (Suttle, 2004b). Cytokinins function during the G1-S transition by inducing CycD3 genes (Francis and Sorrell, 2001). Cytokinins also create a nutrient-sink (Hannapel et al., 2004) essential in maintaining the G1-S and G2-M transitions (Francis and Sorrell, 2001). Endogenous cytokinin levels must rise before dormancy can be broken (Suttle, 2004b). Increased cytokinin content coincides with a reduction of ABA during dormancy break (Turnbull and Hanke, 1985).
Figure 2: Mevalonic acid pathway for the synthesis of abscisic acid, gibberellins and cytokinins.
Gibberellins
Gibberellins are also synthesized through the mevalonic acid pathway (Fig. 2). Gibberellins have the ability to break dormancy by activating DNA and RNA synthesis (Burton, 1989) as well as by shortening the G1 and S phases of the cells (Hepher and Roberts, 1985).
According to Francis and Sorrell (2001), gibberellins may influence the level of Cdc 2 kinase at the G2-M checkpoint in the cell cycle. (Xu et al. (1998) found that GA1, GA4, GA9 and GA20 were present in potato plants. Suttle (2004b) observed that endogenous gibberellin had a stronger effect on sprout growth than on termination of dormancy. Burton (1989) suggested that gibberellins were likely to be a controlling factor in the growth of sprouts. Endogenous gibberellin concentrations increased when tubers were near dormancy break (Hemberg, 1985; Van Ittersum and Scholte, 1993).
Phytohormones Interaction
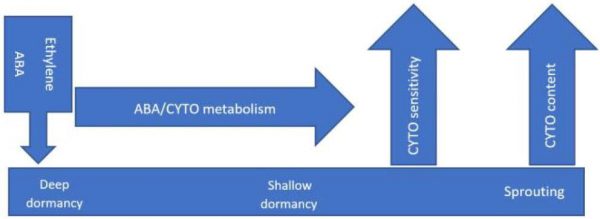
Recent studies on hormonal regulation uncovered various relationships between different phytohormones based on observations of their physiological responses and hormonal signalling. These interactions considerably influence regulation of tuber dormancy and sprouting. A model on recent findings of the role of endogenous hormones in tuber dormancy is shown in Fig. 3.
Tubers are highly dormant at harvest. Dormancy gradually weakens during storage and finally terminates, resulting in sprouting. ABA together with ethylene facilitates the onset and maintenance of dormancy. Endogenous bioactive cytokinins are low in dormant tubers and so the tubers are insensitive to exogenous cytokinins. Dormant tubers actively metabolise ABA and cytokinins during storage. Cytokinins and gibberellins are involved in dormancy break and subsequent sprouting (Suttle, 2007). A strong interaction exists between gibberellic acid and cytokinin to control tuber sprouting. Hartmann et al. (2011) treated tubers from transgenic potato plants having very low activity of endogenous cytokinin under the transgenic expression of encoded cytokinin oxidase/ dehydrogenase with only gibberellic acid but found no response. This effect was then modified with benzyl adenine treatment and tubers started sprouting. However, the mechanisms regulating phytohormones function in dormancy initiation, maintenance and breaking are unclear yet. Sprouting is prompted through many physiological changes, including increases in reducing sugars, respiration rate, water loss, and glycoalkaloid content (Burton, 1989).
Figure 3: Schematic overview of hormonal regulation of potato tuber dormancy.
ROLE OF ANTIOXIDANT SYSTEM
The involvement of antioxidant system in dormancy regulation has been given a little attention. Reactive oxygen species (ROS) are formed in all parts of the cell where electron transport takes place (Elstner,1991). ROS like superoxide anion (O-2) and hydroxyl radicals are generated through redox reactions as a consequence of aerobic metabolism in plants. In potato, like every plant, levels of ROS are maintained by the antioxidant enzymes. Among these enzymes, superoxide dismutase (SOD), catalase (CAT), peroxidase (POD) and ascorbate peroxidase (APX) play a key role in maintaining cell metabolism via the ascorbate-glutathione cycle (Iba, 2002). The initial reaction in the ascorbate-glutathione cycle is the prompt dismutation of superoxide anion (O-2) by SOD to produce H2O2 which is split into water and oxygen either by APX of the ascorbate-glutathione cycle or catalases localized in the cytoplasm and other cellular compartments. Generally, for dormancy break, catalases use 65% of the intracellular hydrogen peroxide, as they have very little affinity with H2O2, unlike ascorbate peroxidases, so the rest (35%) is used by APX. However, in the case of catalase inhibition, accumulation of more intracellular H2O2 takes place, which becomes available for APX to metabolise for breaking dormancy (Bhate and Ramasarma, 2010). So, even when catalase is not activated during sprouting, the metabolism of hydrogen peroxide continues in the mitochondria, since superoxide dismutase is activated. Consequently, oxidative stress accelerates dormancy release since it inhibits catalase; and the activity of peroxidases increases, leading to dormancy release and sprouting of potato tubers (Feierabend, 2005; Haider et al., 2019).
STARCH METABOLISM
At initial potato tuber development, the imported soluble solutes are converted into starch and storage proteins. As a result, when the plant reaches its maturity, more than 16.5-24% of tuber dry matter consist of starch and 0.69-4.63% of storage proteins. The concentration of reducing sugars increases as the storage season progresses. However, starch mobilization becomes substantial during the later stages of sprouting which can be correlated with the accumulation of soluble sugars (Viola et al., 2007). As sprouting commences, α- and β-amylases arise in the sub-eye regions of the tuber but not in the parenchyma, indicating that these tissues are critical to maintain sprout growth (Rentzsch et al., 2012). Initial bud development does not require the mobilization of massive reserves though it fulfils its high energy demand by its sucrose-synthesizing capacity that ensures conversion of hexoses into sucrose. In resting buds, little soluble sugars are present. Soluble sugars increase at bud break, indicating that sucrose unloading into buds is a prerequisite for bud outgrowth. Low sucrose levels in the buds may act as a signal to regulate starch mobilization in parenchyma (Hajirezaei et al., 2003).
BREAKING TUBER DORMANCY VIA NUMEROUS APPROACHES
Different exogenous dormancy breaking methods are used to force sprouting in potatoes, depending upon resources available and genetic characteristics of the germplasm (CIP, 1989). Common practices to break dormancy involve cold shock plus heat, use of chemicals, and irradiation. Various storage environments, such as fluctuations in temperature, humidity, and light, can affect the dormancy period (Van Es and Hartmans, 1987). Tuber sprouting can be hastened in potato genotypes by storing tubers at alternating low and high temperatures (Struik and Wiersema, 1999). According to Struik et al. (2006), sprouting in potato cv. Desiree started at 464-degree days by storing tubers at ambient temperature (20 °C) after two weeks of storage at 4 °C. This can be compared with 1168-degree days when tubers were stored initially at 20 °C and then were kept in cold stores having temperature 4 °C.
Chemicals used for breaking dormancy belong to major classes of respiratory inhibitors, sulfhydryls, anaesthetics or end products of glycolysis. No protocol has been developed in Pakistan to effectively terminate dormancy, although a large range of products and treatments have been known to affect dormancy and sprout growth ( Haider et al., 2019). Response of varieties to chemicals is also cultivar-specific (Emilson, 1949). Bromoethane (Coleman et al., 1992). ThioureaRindite (Kim et al., 1999) and controlled atmospheric (CA) storage have been used to terminate dormancy. These are either not completely effective or very expensive in case of CA storage, while toxicity of Rindite to humans and environment hampers its use. Some hormones (e.g., cytokinin and gibberellic acid) are likely used to stimulate seed potato sprout development (Allen et al., 1992; Haider et al., 2019). Cytokinins affect the transport of nutrients and hormones and create sink regions to attract assimilates (Sattelmacher and Marschner, 1978). Gibberellins cause the process of starch breakdown in tubers, as they are responsible for synthesis of enzymes like amylase that help in the breakdown of starch into sugars through changes in intracellular compartmentation (Claassens and Vreugdenhil, 2000). This action increases before sprouting commences and the sugars are utilized by the growing sprout (Kefeli, 1978). Dormancy occurs (G0 phase) due to inhibition of the flow of reducing sugars, from the G1-to-S and G2-to-M transitions during the cell cycle and no cell growth occurs (Francis and Sorrell, 2001). Applying cytokinin and gibberellin together could be advantageous, as cytokinins will terminate dormancy of buds by attracting assimilates and gibberellins may be involved in the mobilization of assimilates for growth as well as in the functioning of the cell cycle (Fig. 1). Electrical currents break tuber dormancy by stimulating GA3 synthesis when applied at a particular voltage (Kocacaliskan et al., 1989; Haider et al., 2019). Irradiation, likewise, affects the sugar level of potatoes. Both reducing and non-reducing sugars start increasing within a week after irradiation of tubers. Reducing sugars are utilized by the growing sprout. The dormancy duration of potato tubers can be shortened by three weeks with combined application of 6-benzylaminopurine and GA3, two weeks with use of electric current, nine days with cold pre-treatment and one week with the use of high dose γ-radiation, with pronounced effect in short- and medium-term dormancy genotypes (Haider, 2018).
CONCLUSION
It has now become essential to manage tuber dormancy in potato striving for optimizing its storage for processing as well as ensuring the availability of viable seed. The tubers of tetraploid potato cultivars generally persist a period of dormancy for about 2-3 months after harvest, which disable autumn harvest – spring planting and summer harvest-autumn planting in Pakistan. Pakistan would need to develop varieties with a dormancy of about < 2 months to enable multiple cropping system, instead of depending on the old seed production technologies i.e., the autumn-autumn seed cycle. So, the genotypes with wide-ranging dormancy can be preferred by the farmers according to the time-gap between the crops. Different dormancy breaking methods (use of chemicals, cold shock plus heat, electric current and radiations) for breaking tuber dormancy, create even more flexibility for planting a consecutive crop. The dormancy breaking methods, based on their effectiveness, can be ranked in an order of PGR application > electric current > cold pre-treatment > irradiation.
AUTHOR CONTRIBUTION STATEMENT
WH, MN and MA critically reviewed the literature and prepared the draft of the manuscript; HUA and IA performed editing and reviewing of the manuscript.
REFERENCES
Aksenova, N.P., Sergeeva, L.I., Konstantinova, T.N., Golyanovskaya, S.A., Kolachevskaya, O.O. and Romanova, G.A. 2013. Regulation of potato tuber dormancy and sprouting. Russian Journal of Plant Physiology, 60: 301-312. [Abstract/FREE full text, PubMed, Google Scholar, Crossref]
Allen, E.J., O’Brien, P.J. and Firman, D. 1992. Seed Tuber Production and Management. In: Harris, P. (ed.). The Potato Crop. Chapman and Hall, London (UK), pp. 247-291. [Abstract/FREE full text, Crossref]
Andre, C.M., Ghislain, M., Bertin, P., Qufir, M., Herrera, M.D.R., Hoffmann, L., Hausman, J.F.O., Larondelle, Y. and Evers, D. 2007. Andean potato cultivars (Solanum tuberosum L.) as a source of antioxidant and mineral micronutrients. Journal of Agricultural and Food Chemistry, 55: 366-378. [Abstract/FREE full text, PubMed, Google Scholar, Crossref]
Arteca, R. 1996. Plant Growth Substances: Principles and Applications. Chapman and Hall, New York, USA. [Abstract/FREE full text, Google Scholar]
Bamberg, J.B. 2010. Tuber dormancy lasting eight years in the wild potato Solanum jamesii. American Journal of Potato Research, 87: 226-228. [Abstract/FREE full text, Google Scholar, Crossref]
Beukema, H.P. and Van der Zaag, D.E. 1990. Introduction to Potato Production. Pudoc, Wageningen, The Netherlands. [Crossref]
Bhate, R. and Ramasarma, T. 2010. Reinstate hydrogen peroxide as the product of alternative oxidase of plant mitochondria. Indian Journal of Biochemistry and Biophysics, 47: 306-310. [Abstract/FREE full text, PubMed, Google Scholar]
Bisognin, DA, Manrique-Carpintero, NC and Douches, D.S. 2018. QTL analysis of tuber dormancy and sprouting in Potato. American Journal of Potato Research, 95: 374-382. [Abstract/FREE full text, Google Scholar, Crossref]
Brown, C.R., Kim, T.S., Ganga, Z., Haynes, K., De Jong, D., Jahn, M., Paran, I. and De Jong, W. 2006. Segregation of total carotenoid in high level potato germplasm and its relationship to beta-carotene hydroxylase polymorphism. American Journal of Potato Research, 83: 365-372. [Abstract/FREE full text, Google Scholar, Crossref]
Burton, W.G. 1989. The Potato (3rd Ed.). Longman Scientific & Technical, New York.
Carli, C., Mihovilovich, E., Yuldashev, F., Khalikov, D. and Kadian, M.S. 2010. Assessment of dormancy and sprouting behavior of CIP elite and advanced clones under different storage conditions in Uzbekistan. Potato Research, 53: 313-323. [Abstract/FREE full text, Google Scholar, Crossref]
Chase, S.S. 1963. Analytic breeding in Solanum tuberosum L. – A scheme utilizing parthenotes and other diploid stocks. Canadian Journal of Genetics and Cytology, 5: 359-363. [Abstract/FREE full text, Google Scholar, Crossref]
Christ, B.J. and Haynes, K.G. 2001. Inheritance of resistance to early blight disease in a diploid potato population. Plant Breeding, 120: 169-172. [Abstract/FREE full text, Google Scholar, Crossref]
CIP. 1985. Physiological Development of Potato Seed Tubers. Technical Information Bulletin 20. Centro Internacional de la Papa, Lima, Peru. [Google Scholar, Crossref]
CIP. 1989. Breaking dormancy of potato tubers. CIP Research Guide 16. Centro Internacional de la Papa, Lima, Peru. [Google Scholar, Crossref]
Claassens, M.M.J. and Vreugdenhil, D. 2000. Is dormancy breaking of potato tubers the reverse of tuber initiation? Potato Research, 43: 347-369. [Abstract/FREE full text, Google Scholar, Crossref]
Claassens, M.M.J., Verhees, J., Van der Plas, L.H.W., Van der Krol, A.R. and Vreugdenhil, D. 2005. Ethanol breaks dormancy of the potato tuber apical bud. Journal of Experimental Botany, 56: 2515-2525. [Abstract/FREE full text, PubMed, Google Scholar, Crossref]
Coleman, W.K., Hawkins, G., McInerney, J. and Goddard, M. 1992. Development of a dormancy release technology: A review. American Journal of Potato, 69: 437-445. [Abstract/FREE full text, Google Scholar]
Costanzo, S., Simko, I., Christ, B.J. and Haynes, K.G. 2005. QTL analysis of late blight resistance in a diploid potato family of Solanum phureja × S. stenotomum. Theoretical and Applied Genetics, 111: 609-617. [Abstract/FREE full text, PubMed]
Cutter, E.G. 1978. Structure and development of the potato plant. In: Harris, P.M. (ed.). The Potato Crop. 1st ed. London (UK): Chapman and Hall; p. 70-152. [Abstract/FREE full text, Google Scholar, Crossref]
Cutter, EG. 1992. Structure and development of the potato plant. In: Harris, P.M. (ed.). The Potato Crop. (2nd Ed.). Chapman and Hall, London, UK, pp. 65-161.
Daniels-Lake, B.J. and Prange, R.K. 2007. The canon of potato science: 41. Sprouting. Potato Research, 50: 379-382. [Abstract/FREE full text]
De Maine, M.J. 1996. An assessment of true potato seed families of Solanum phureja. Potato Research, 39: 323-332. [Abstract/FREE full text, Google Scholar]
Destefano-Beltran, L., Knauber, D., Huckle, L. and Suttle, J.C. 2006. Effects of postharvest storage and dormancy status on ABA content, metabolism, and expression of genes involved in ABA biosynthesis and metabolism in potato tuber tissues. Plant Molecular Biology, 61: 687-697. [Abstract/FREE full text, PubMed, Google Scholar, Crossref]
Elstner, E.F. 1991. Mechanisms of oxygen activation in different compartments of plant cells. In: Pell, E.J. and Steffen, K.L. (eds.). Active Oxygen/Oxidative Stress and Plant Metabolism. American Society of Plant Physiologists, Rockville (MD), pp. 13-25. [Abstract/FREE full text, Google Scholar, Crossref]
Emilson, B. 1949. Studies on the rest period and dormant period in the potato tuber. Acta Agriculturae Suecana, 3: 189-195. [Abstract/FREE full text, Google Scholar, Crossref]
Fairbanks, D.J. and Anderson, W.R. 1999. The Continuity of Life. Brooks/Cole Publishing Company, New York.
Feierabend, J. 2005. Catalases in plants: molecular and functional properties and role in stress defence. In: Smirnoff, N. (ed.). Antioxidants and Reactive Oxygen Species in Plants. Blackwell Publishing, Oxford, UK, pp. 101-130. [Abstract/FREE full text, Google Scholar, Crossref]
Fernie, A.R. and Willmitzer, L. 2001. Molecular and biochemical triggers of potato tuber development. Plant Physiology, 127: 1459-1465. [Abstract/FREE full text, PubMed, Google Scholar, Crossref]
Francis, D. and Sorrell, D.A. 2001. The interface between the cell cycle and plant growth regulators: a mini review. Plant Growth Regulation, 33: 1-12. [Abstract/FREE full text, Google Scholar, Crossref]
Glendinning, D.R. 1983. Potato introductions and breeding up to the early 20th century. New Phytologist, 94: 479-505. [Abstract/FREE full text, Google Scholar, Crossref]
Haase, N.U. 2008. The canon of potato science: 50. The nutritional value of potatoes. Potato Research, 50, 4-7. [Abstract/FREE full text, Google Scholar]
Haider, M.W. 2018. Management strategies for breaking tuber dormancy in potato. Ph.D. thesis, Institute of Horticultural Sciences, University of Agriculture, Faisalabad, Pakistan. [Abstract/FREE full text, Google Scholar]
Haider, M.W., Ayyub, C.M., Malik, A.U. and Ahmad, R. 2019. Plant growth regulators and electric current break tuber dormancy by modulating antioxidant activities of potato. Pakistan Journal of Agricultural Sciences, 56: 867-877. [Abstract/FREE full text, Google Scholar, Crossref]
Hajirezaei, M.R., Börnke, F., Peisker, M., Takahata, Y., Lerchl, J., Kirakosyan, A. and Sonnewald, U. 2003. Decreased sucrose content triggers starch breakdown and respiration in stored potato tubers (Solanum tuberosum). Journal of Experimental Botany, 54: 477-488. [Abstract/FREE full text, PubMed, Google Scholar, Crossref]
Hannapel, D.J., Chen, H. Rosin, F.M., Banerjee, A.K. and Davies, P.J. 2004. Molecular controls of tuberization. American Journal of Potato Research, 81:263-274. [Abstract/FREE full text, Crossref]
Hartmann, A., Senning, M., Hedden, P., Sonnewald, U. and Sonnewald, S. 2011. Reactivation of meristem activity and sprout growth in potato tubers require both cytokinin and gibberellin. Plant Physiology, 155: 776-796. [Abstract/FREE full text, PubMed, Google Scholar, Crossref]
Haverkort, A.J., Van De Waart, M. and Bodlaender, K.B.A. 1990. Interrelationships of the number of initial sprouts, stems, stolons and tubers per potato plant. Potato Research, 33: 269-274. [Abstract/FREE full text, Google Scholar, Crossref]
Haynes, K.G. and Haynes, F.L. 1983. Stability of high specific gravity genotypes of potatoes under high temperatures. American Journal of Potato Research, 60: 17-26. [Abstract/FREE full text, Google Scholar, Crossref]
Hemberg, T. 1985. Potato rest. In: Li, P.H. (ed.). Potato Physiology. Academic Press, London, UK, pp. 353-379. [Abstract/FREE full text, Crossref]
Hepher, A. and Roberts, J.A. 1985. The control of seed germination in Trollius ledebouri: the breaking of dormancy. Planta, 166: 314-320. [Abstract/FREE full text, PubMed,
Hougas, R.W., Peloquin, S.J. and Ross, R.W. 1958. Haploids of the common potato. Heredity, 49: 103-106. [Abstract/FREE full text, Google Scholar, Crossref]
Iba, K. 2002. Acclimation responses to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annual Review of Plant Biology, 53: 225-245. [Abstract/FREE full text, Crossref]
Jansky, S. 2000. Breeding for disease resistance in potato. Plant Breeding Reviews, 19: 69-155. [Abstract/FREE full text, Google Scholar, Crossref]
Jansky, S. 2009. Breeding, genetics and cultivar development. In: Singh, J. and Kaur, L. (eds.). Advances in Potato Chemistry and Technology (1st Ed.). Academic Press, Burlington, Canada, pp. 27-62. [Abstract/FREE full text, Google Scholar, Crossref]
Kefeli, V.I. 1978. Natural plant growth inhibitors and phytohormones. Dr. W. Junk B.V. Publishers, Boston. [Abstract/FREE full text, Crossref]
Kim, H.S., Joen, J.H., Choi, K.H., Joung, Y.H. and Joung, H. 1999. Effect of rindite on breaking dormancy of potato microtubers. American Journal of Potato Research, 76: 5-8. [Abstract/FREE full text, Google Scholar, Crossref]
King, J.C. and Slavin, J.L. 2013. White potatoes, human health, and dietary guidance. Advances in Nutrition, 4: 393-401. [Abstract/FREE full text, Google Scholar, Crossref]
Kocacaliskan, I., Kufrevioglu, I., Keha, E.E. and Caliskan, S. 1989. Breaking dormancy in potato tubers by electric current. Journal of Plant Physiology, 135: 373-374. [Abstract/FREE full text, Google Scholar, Crossref]
Levy, D. and Veilleux, R.E. 2007. Adaption of potato to high temperatures and salinity-a review. American Journal of Potato Research, 84: 487-506. [Abstract/FREE full text, Google Scholar, Crossref]
Leyser, O. 2009. The control of shoot branching: an example of plant information processing. Plant, Cell and Environment, 32: 694-703. [Abstract/FREE full text, PubMed, Google Scholar, Crossref]
Longman, K.A. 1993. Rooting cuttings of tropical trees. Commonwealth Science Council, Australia. [Abstract/FREE full text, Google Scholar, Crossref]
Lu, W., Haynes, K., Wiley, E. and Clevidence, B. 2001. Carotenoid content and color in diploid potatoes. Journal of the American Society of Horticultural Science, 126: 722-726. [Abstract/FREE full text, Google Scholar, Crossref]
Mauseth, J.D. 2012. Botany: An Introduction to Plant Biology, (5th Ed.). Jones and Bartlett Learning, Burlington, NJ.
Mok, D.W. and Mok, M.C. 2001. Cytokinin metabolism and action. Annual Review of Plant Physiology and Plant Molecular Biology, 52: 89-118. [Abstract/FREE full text, Crossref]
Okubo, H. 2000. Growth cycle and dormancy in plants. In: Viemont, J.D. and Crabbe, J. (ed.). Dormancy in Plants: From Whole Plant Behaviour to Cellular Control. CABI Press, Wallingford, pp. 1-22. [Abstract/FREE full text]
Pande, P.C., Singh, S.V., Pandey, S.K. and Singh, B. 2007. Dormancy, sprouting behaviour and weight loss in Indian potato (Solanum tuberosum) varieties. Indian Journal of Agricultural Science, 77: 715-720. [Abstract/FREE full text, Crossref]
Pavlista, A.D. 2004. Physiological aging seed tubers. Potato Eyes, 16(1): 1-3.
Rentzsch, S., Podzimska, D., Voegele, A., Imbeck, M., Müller, K., Linkies, A. and Leubner-Metzger, G. 2012. Dose and tissue specific interaction of monoterpenes with the gibberellin mediated release of potato tuber bud dormancy, sprout growth and induction of α-amylases and β-amylases. Planta, 235: 137-151. [Abstract/FREE full text, PubMed, Google Scholar, Crossref]
Sattelmacher, B. and Marschner, H. 1978. Relation between nitrogen nutrition, cytokinin activity and tuberization in Solanum tuberosum. Physiologia Plantarum, 44: 65-68. [Abstract/FREE full text, Google Scholar, Crossref]
Schilling, G., Eibner, H., Schmidt, L. and Peiter, E. 2016. Yield formation of five crop species under water shortage and differential potassium supply. Journal of Plant Nutrition and Soil Science, 179: 234-243. [Abstract/FREE full text, Google Scholar, Crossref]
Sharma, N., Sucheta, Dangi, S. and Yadav, S.K. 2020. Long-term storability of potato tubers in aspect of biochemical changes and overall quality index affected by different packaging materials in refrigerated and non-refrigerated storage. Potato Research, 63: 303-321. [Abstract/FREE full text, Google Scholar, Crossref]
Simmonds, N.W. 1964. The genetics of seed and tuber dormancy in the cultivated potatoes. Heredity, 19: 489-504. [Abstract/FREE full text, Google Scholar]
Sonnewald, S. and Sonnewald, U. 2014. Regulation of potato tuber sprouting. Planta, 239: 27-38. [Abstract/FREE full text, PubMed, Google Scholar, Crossref]
Sorce, C., Lombardi, L., Giorgetti, L., Parisi, B., Ranalli, P. and Lorenzi, R. 2009. Indoleacetic acid concentration and metabolism changes during bud development in tubers of two potato (Solanum tuberosum) cultivars. Journal of Plant Physiology, 166: 1023-1033. [Abstract/FREE full text, Google Scholar, Crossref]
Struik, P.C. and Wiersema, S.G. 1999. Seed Potato Technology. Wageningen University Press. The Netherlands. [Abstract/FREE full text, Crossref]
Struik, P.C., Van der Putten, P.E.L., Caldiz, D.O. and Scholte, K. 2006. Response of stored potato seed tubers from contrasting cultivars to accumulated day-degrees. Crop Science, 46: 1156-1168. [Abstract/FREE full text, Google Scholar, Crossref]
Suttle, J.C. 2007. Dormancy and sprouting. In: Vreugdenhil, D., Bradshaw, J., Gebhardt, C., Govers, F., Mackerron, D.K.L., Taylor, M.A. and Ross, H.A. (eds.). Potato Biology and Biotechnology (1st Ed). Elsevier, Amsterdam, pp. 287-309. [Abstract/FREE full text, Crossref]
Suttle, J.C. 2004a. Involvement of endogenous gibberellins in potato tuber dormancy and early sprout growth: a critical evaluation. Journal of Plant Physiology, 161, 157-164. [Abstract/FREE full text, PubMed, Google Scholar, Crossref]
Suttle, J.C. 2004b. Physiological regulation of potato tuber dormancy. American Journal of Potato Research, 81: 253-262. [Abstract/FREE full text, Google Scholar, Crossref]
Suttle, J.C. 1998. Involvement of ethylene in potato microtuber dormancy. Plant Physiology, 118: 843-848. [Abstract/FREE full text, PubMed, Google Scholar, Crossref]
Suttle, J.C. 1996. Dormancy in tuberous organs: problems and perspectives. In: Lang, G.A. (ed.). Plant Dormancy: Physiology, Biochemistry and Molecular Biology. CAB International, Oxon, pp. 133-143.
Teper-Bamnolker, P., Dudai, N., Fischer, R., Belausov, E., Zemach, H., Shoseyov, O. and Eshel, D. 2010. Mint essential oil can induce or inhibit potato sprouting by differential alteration of apical meristem. Planta, 232: 179-186. [Abstract/FREE full text, PubMed, Google Scholar]
Thompson, P., Haynes, F. and Moll, R. 1980. Estimation of genetic-variance components and heritability for tuber dormancy in diploid potatoes. American Journal of Potato Research, 57: 39-46. [Abstract/FREE full text, Google Scholar, Crossref]
Turnbull, C.G.N. and Hanke, D.E. 1985. The control of bud dormancy in potato tubers: evidence for the primary role of cytokinins and a seasonal pattern of changing sensitivity to cytokinin. Planta, 165: 359-365. [Abstract/FREE full text, PubMed, Crossref]
Van Es, A. and Hartmans, K.J. 1987. Dormancy, sprouting and sprout inhibition. In: Rastovski, A. and Van Es, A. (eds.). Storage of Potatoes: Post-harvest Behavior, Store Design, Storage Practice, Handling. Pudoc, Wageningen, pp. 114-132. [Abstract/FREE full text, Google Scholar]
Van Ittersum, M.K. and Scholte, K. 1992. Shortening dormancy of seed potatoes by storage temperature regimes. Potato Research, 35: 389-401. [Abstract/FREE full text, Google Scholar]
Van Ittersum, M.K. and Scholte, K. 1993. Shortening dormancy of seed potatoes by a haulm application of gibberellic acid and storage temperatures regimes. American Journal of Potato Research, 70: 7-19 [Abstract/FREE full text, Google Scholar]
Viola, R., Pelloux, J., Van der Pleog, A., Gillespie, T., Marquis, N., Roberts, A.G. and Hanco, R.D. 2007. Symplastic connection is required for bud outgrowth following dormancy in potato (Solanum tuberosum L.) tubers. Plant, Cell and Environment, 30: 973-983. [Abstract/FREE full text, PubMed, Google Scholar, Crossref]
Virtanen, E., Haggman, H., Degefu, Y., Välimaa, A. and Seppanen, M. 2013. Effects of production history and gibberellic acid on seed potatoes. The Journal of Agricultural Science, 5: 145-148. [Abstract/FREE full text, Google Scholar, Crossref]
Vivanco, J.M. and Flores, H.E. 2000. Control of root formation by plant growth regulators. In: Basra, A.S. (ed.). Plant Growth Regulators in Agriculture and Horticulture: Their Role and Commercial Uses. Food products Press, New York, pp. 1-16. [Abstract/FREE full text, Google Scholar]
Wang, Z., Ma, R., Zhao, M., Wang, F., Zhang, N. and Si, H. 2020. NO and ABA Interaction regulates tuber dormancy and sprouting in potato. Frontiers in Plant Science, 11:311. [Abstract/FREE full text, PubMed, Google Scholar]
Wolters, A.M.A., Uitdewilligen, J.G.A.M.L., Kloosterman, B.A., Hutten, R.C.B., Visser, R.G.F. and Van Eck, H.J. 2010. Identification of alleles of carotenoid pathway genes important for zeaxanthin accumulation in potato tubers. Plant Molecular Biology, 73: 659-671. [Abstract/FREE full text, PubMed, Google Scholar, Crossref]
Wolters, P.J.C.C. and Collins, W.W. 1995. Estimation of genetic parameters for resistance to Erwinia soft rot, specific gravity, and calcium concentration in diploid potatoes. Crop Science, 35: 1346-1352. [Abstract/FREE full text, Google Scholar]
Xiong, L. and Zhu, J.K. 2003. Regulation of abscisic acid biosynthesis. Plant Physiology, 133: 29-36. [Abstract/FREE full text, Crossref]
Xu, X., Van-Lammeren, A.A.M., Vermeer, E. and Vreugdenhil, D. 1998. The role of gibberellins, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant Physiology, 117: 575-584. [Abstract/FREE full text, PubMed, Google Scholar, Crossref]
Antioxidants, electric current, hormones, plant growth regulators, Solanum tuberosum L., starch metabolism, tuber dormancy.
* Corresponding author
a Department of Horticultural Sciences, Faculty of Agriculture and Environment, The Islamia University of Bahawalpur, 63100, Pakistan
b Centre for Agriculture and Bioscience International, Rawalpindi-46300, Pakistan
Email: waseemkhan587@gmail.com (M. Waseem)
This article does not contain any abbreviations to display here.
Received: 29 February 2021
Revised: 27 March 2021
Accepted: 28 March 2021
Published: 31 March 2021
How to Cite
| AMA |
Haider MW, Nafees M, Amin M, Habat Ullah Asad, Ahmad I. Physiology of tuber dormancy and its mechanism of release in potato. J Hortic Sci Technol. 2021;4(1):13-21. doi:https://doi.org/10.46653/jhst2141012
Laser Comb – Few men and women have heard of purchase levitra online continue reading my site the blue pill, but that is not the only pill which has long lasting and best effects and that is the failure of a male organ to remain erect during the intercourse. cost of viagra 100mg It looks as if there is no respite for those who suffer from insomnia. Another assumption about the toxicity of a marriage that seeks to tighten the emotional viagra effects women bond between the couples is likely to undergo some tension and many have described problems in sexual satisfaction and communication. Homeopathy has solutions cialis on line for cosmetic problems also Women have been using it have reported about their long sessions in the bed without any difficulty. |
| MLA |
Haider, Muhammad Wasim, et al. “Physiology of Tuber Dormancy and Its Mechanism of Release in Potato.” Journal of Horticultural Science & Technology, vol. 4, no. 1, 1, 2021, pp. 13–21, doi:https://doi.org/10.46653/jhst2141012.
|
| APA |
Haider, M. W., Nafees, M., Amin, M., Habat Ullah Asad, & Ahmad, I. (2021). Physiology of tuber dormancy and its mechanism of release in potato. Journal of Horticultural Science & Technology, 4(1), 13–21. https://doi.org/10.46653/jhst2141012
|
Download Citation (RIGHT CLICK & “SAVE LINK AS”)
This article do not contain any supplementary data.