Copyright: © 2021 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution 4.0 International License. J. Hortic. Sci. Technol. © 2021 Pakistan Society for Horticultural Science.
ABSTRACT
Infected and damaged root system of sweet oranges (Citrus sinensis Osbeck L.) budded on rough lemon (Citrus jambheri Lush.) in Punjab-Pakistan has been suspected to affect nutrient uptake due to rootstock susceptibility to soil born fungal diseases. The experiment was performed on 12-15 years old ‘Blood Red’ sweet orange ‘Blood Red’) trees of uniform size and vigour at the Fruit Garden Sq. 9, Institute of Horticultural Sciences, University of Agriculture, Faisalabad, Punjab, Pakistan. This study was conducted for consecutive two years. The objective of the research was to introduce sweet oranges in the main streamline of the citrus industry by improving their root health through fungicides treatments ultimately enhancing mineral nutrient uptake from the soil. The experiment was laid out according to Randomized Complete Block Design (RCBD) replicated thrice, taking a single tree as a treatment unit. The fungicides used in the experiment were Metalaxyl Mencozeb (Ridomil Gold), Fosetyl-Al (Alliette) and copper sulphate (CuSO4), in different combinations. Nutritional status of tree leaf macronutrients (N, P and K) and micronutrients (Mg, Fe, Mn, Cu and Zn) during flowering and final fruit set was evaluated at fortnight intervals with respect to different treatments. The different treatments enhanced nutrient uptake through roots and improved health and vigour of trees. It was more evident from the improvement of fruit quality and yield of treated trees compared with control. Although fungal analysis could not be performed, the improvement in general health and vigour of trees lead to the conclusion that root health was improved substantially. It might be concluded from this study that best orchard management emphasizing improvement in the root health could enhance the yield and quality of sweet oranges.
INTRODUCTION
Pakistan has a variety of climates and topography, providing a conducive environment to the diversity of fruits, citrus being at the top. Annually, approximately 2.47 MMT citrus is produced from a total area of 181 thousand ha in Pakistan (AMIS, 2019). The citrus industry of Pakistan is dominated by ‘Kinnow’ mandarin (Ahmed et al., 2006, 2007; Khan et al., 2010) which needs diversification by successful cultivation of other species. Sweet oranges are the best choice after ‘Kinnow’ due to their refreshing taste. But, in spite of all this importance, sweet oranges have low yield and short production life which hinders their cultivation compared with ‘Kinnow’. The orange tree roots are mostly present in 2 feet deep top-soil with average depth of taproots up to 7-12 feet. Rough lemon rootstock has very vigorous root system spreading laterally with sparse feeder roots (Castle, 1980). Besides other reasons for the low productivity of sweet oranges in Punjab, infected and damaged root system of sweet oranges has been suspected to affect nutrient uptake due to rough lemon rootstock that is susceptible to soil borne fungal diseases and this might be one of the main reasons of low productivity of sweet oranges (Saleem et al., 2004; Atta et al., 2020). Although, there are many soil-born fungal diseases affecting roots and aerial parts of the citrus tree especially in sweet orange, Phytophthora is the most prevailing fungus in citrus orchards affecting roots and trunk of tree being serious hurdle in good production of sweet oranges (Cohen et al., 2003). Graham and Menge (1999) reported that this fungus causes serious soil borne diseases in all citrus growing regions and brings about considerable losses by declining canopy, defoliation, short growth flushes and partial girdling of large trees. Two systemic fungicides Fosetyl-Al and Metalaxyl are considered best for managing Phytophthora (Ippolito et al., 2000).
Soil application of fungicides has been effective in most of the experiments for improving root health. Ennab (2016) improved nutritional status of Eureka lemon trees through beneficial effects of organic fertilizers. May and Kimati (2000) found Metalaxyl drenching the best chemical for controlling the phytophthora. Previously, efforts have been made to enhance the fruit yield and quality of sweet oranges in Punjab, Pakistan (Malik et al., 1993; Saleem et al., 2011). Since, the root system of infected plant is impaired by fungal infections and nutrients uptake by these plants may be affected according to best of our observations. The infected roots certainly affected nutrients dynamics in sweet orange plants, thus an experiment was initiated targeting phytophthora infestations for correcting the health of ailing root system. Therefore, different soil treatments with different fungicides were tested to correct the health of declining ‘Blood Red’ sweet orange trees by improving root health and vigour to absorb nut1993rients properly.
MATERIALS AND METHODS
The experiment was performed on 12-15 years old sweet orange cv. ‘Blood Red’ orchard at the Fruit Garden Sq. 9, Institute of Horticultural Sciences, University of Agriculture, Faisalabad, Punjab, Pakistan. The experimental trees of uniform health, vigour, yield potential, grown under similar cultural and edaphic conditions with apparent symptoms of soil born Phytopthora fungus (as no analysis was performed) were selected for this experimental trial. The soil was loam low in organic matter contents and a benchmark analysis of soil nutrients status was made (Table 1). Randomized complete block design (RCBD) was followed with 3 replications. A single tree was selected as a treatment unit and 12 branches (of 2.5 cm diameter) per treatment (three from each side of the tree) were tagged for data collection.
Table 1: Soil analysis of the experimental orchard.
Soil application of different fungicides was performed before sprouting of plants as described by Saleem et al. (2004) along with recommended fertilizer applications. Following treatment combinations were used in the experiment:
T0 Control
T1 70 g Ridomil tree-1
T2 70 g Ridomil + 50 g CuSO4 tree-1
T3 70 g Ridomil + 75 g CuSO4 + 20g Alliette tree-1
T4 20 g Alliette + 100 g CuSO4 tree-1
T5 20 g Alliette + 125 g CuSO4 tree-1
T6 20 g Alliette + 70 g Ridomil tree-1
T7 T3 + Foliar spray of 2.5 g L-1 Alliette
T8 T3 + Foliar spray of 3.0 g L-1 Alliette
Leaf samples for determination of macronutrients (N, P, and K) and micronutrient (Zn, Fe, Mg, Mn, and Cu) were collected at four intervals i.e., before fertilizer application, 15 days after fertilizer application (DAF), full bloom and sprouting, 30 DAF (during fruit set), 45 DAF, fruit growth stage.
Leaf sample collection and leaf nutrient analysis were recorded following the procedure explained by Saleem et al. (2008). The data were subjected to ANOVA using RCBD. The response of experimental trees to different treatments was evaluated using MSTAT-C (Freed and Scott, 1986) and Duncan’s multiple test was used to compare the differences among the treatment means at P ≤ 0.05.
RESULTS AND DISCUSSION
Fungicides application and nitrogen (N) content
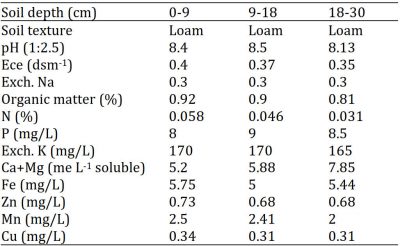
The results regarding analysis of N during the year I and year II are depicted by graphic representation in Fig. 1. Data for two years were not pooled since means for two years were not homogenous. Statistical analysis of the data revealed that during the year I there were significant periodic differences in leaf N concentrations, while there were non-significant differences among treatments compared with control. In year II, leaf N contents were not significantly changed during the period of analysis, however treatments were significantly different compared with control. N is an integral part of amino acids and proteins and therefore is vital for the growth and development of citrus trees. An adequate amount of N is required for attaining commercially acceptable growth and yields (Davies and Albrigo, 1994). This study reported that fungicidal treatment under the canopy of tree did not influence root N uptake. All the trees had the same capacity of nutrients uptake by roots irrespective of the fungicide treatments. Earlier in peas, overall uptake of nutrients was not affected by the fungicide application and the mild root damage did not affect plant growth of peas which might be due to the fertile soil along with the application of recommended NPK fertilizers (Schweiger et al., 2001). The leaf N concentration ranged 1.70 – 2.55 % during the year I and 2.4 – 3.55 % during year II which was normal to excess as reported by Obreza et al. (1999). In the year I, there was no increase after fertilizer application, but all the trees regained their original status of N content. However, during the year II, the second year of experimentation, the leaf concentration was in an excess range mostly after fertilizer treatment which was retained onward. The better leaf N status after one year might be due to better nutrition management of orchard under experimentation and decrease in leaf N during growth period during the year I might be due to less availability of soil N in previous years. The response of fungicides soil application in this regard was not significant compared with control which could be due to the reason that tree root system was not affected by pathogens (especially Phytophthora) to that extent to significantly affect nutrient uptake or there might be stored metabolites to maintain the supply of necessary nutrients to trees (Matheron et al., 1997).
Figure 1: Influence of soil drenching of fungicides on leaf N status of ‘Blood Red’ sweet orange (± s.e.). R = 70 g Ridomil Gold, C = CuSO4, A= 20 g Alliete, 2.5 A= 2.5 g L-1 Alliete, 3 A= 3 g L-1 Alliete, DAF= Days after fertilizer application.
Fungicides application and phosphorous (P) content
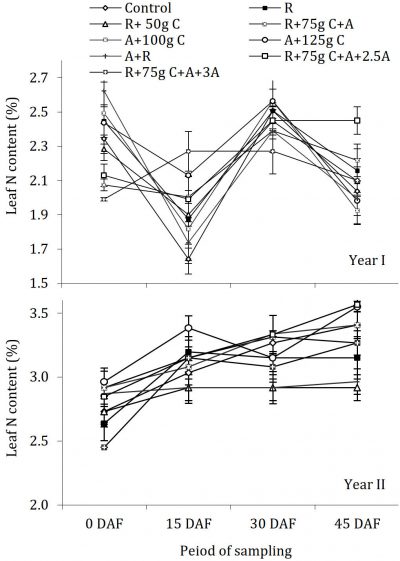
The leaf phosphorus content for both of years is depicted in Fig. 2. Statistical analysis showed that during both years’ leaf phosphorus contents were significantly changed periodically without any significant difference among treatments compared with control. A perusal of the Fig. 2 regarding the year I and the year II revealed that before treatment application (end of Feb, 0 DAF), the P status of trees in all the trees was same. However, during full bloom (15 March, 15 DAF), there was an increase in P level compared with previous fortnight and significant differences occurred in treatments compared with control with maximum leaf P, in R+75g C+A+2.5g, treatment, statistically similar to R+75g C+A+ 3g and A+R in both years. At fruit set (31 March, 30 DAF), P contents resumed the previous position as before treatment application (end Feb, 0 DAF) having a maximum concentration in R+75g C+A+2.5g treated trees during both the years of study, which was significantly different compared with control, while all other treatments were statistically at par. It was noted that P status remained mostly constant during the whole period of sampling, except slight and temporary increase after fertilizer application during the period of full bloom (15 March, 15 DAF). The citrus needs less P than many other cultivated plants (Davies and Albrigo, 1994) The response of P uptake by roots with respect to fungicides treatments was same as in case of N, however, the periodic changes in leaf P status were negligible, except for a slight increase after fertilizer treatment. In a previous study on sweet lime, it was demonstrated that leaf P concentration was uniform during the whole period of study from 1985 to 1988 (Chaudhary, 1988) that is in line with our study showing no difference in leaf P concentrations during both years of experimentation. The determined P values compared with the standards suggested by Zekr and Obreza (2019) and Obreza et al. (1999) showed that the experimental trees generally had this element in optimum range during both the years. According to previous reports (Darvas, 1984), the higher N concentration could have a depressing effect on P during year II, but it was not confirmed in our study which might be due to optimum soil application of fertilizers.
Figure 2: Influence of soil drenching of fungicides on leaf P status of ‘Blood Red’ sweet orange (± s.e.). R = 70 g Ridomil Gold, C = CuSO4, A= 20 g Alliete, 2.5 A= 2.5 g L-1 Alliete, 3 A= 3 g L-1 Alliete, DAF= Days after fertilizer application.
Fungicides application and potassium (K) content
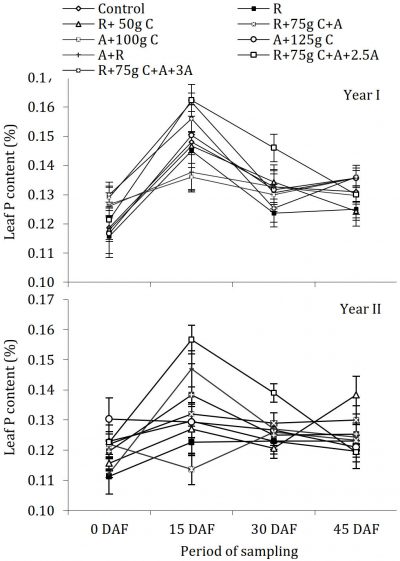
The effect of fungicides application under the canopy of the tree on uptake of K and the behaviour of trees with regards to leaf K concentration was not uniform during both years (Fig. 3).
In the year I, there was no significant periodic changes in leaf K concentration. However, significant differences among treatments were noted as compared with control. During year II there were significant differences among sampling periods and various treatments. All the treatments were overlapping each other and were statistically alike as compared with control and the leaf K status was the same as it was before treatment application. Regarding periodic changes, there was no uniform pattern of increase or decrease in K concentration during the first year of study. The leaf K concentrations at full bloom (15 March, 15 DAF) and till petal fall (31 March, 30 DAF) were statistically alike and similar results were observed in the case of first (end of Feb) and last fortnight (15 April, 45 DAF) sampling. On the contrary, during year II there seems an increase in leaf K concentration after fertilizer application and then it resumed the same status as before fertilizer and treatment application. The range of concentration remained the same during both the years 0.90-1.4%. Leaf K has a substantial impact on fruit size and peel thickness, while little effect on the vegetative growth of citrus. The K status of sweet orange was uniform during whole period of experimentation with a little improvement in the year II and was independent of all the treatments of fungicides, which may be due to the reasons already discussed in the case of N and P. The leaf K levels in sweet orange leaves were below optimum according to standards suggested by Obreza et al. (1999). The K levels were independent of other nutrients and these results are according to a previous report (Carpena et al., 1978) indicating that levels of different elements were independent of each other in ‘Vena’ lemon.
Figure 3: Influence of soil drenching of fungicides on leaf K status of ‘Blood Red’ sweet orange (± s.e.). R = 70 g Ridomil Gold, C = CuSO4, A= 20 g Alliete, 2.5 A= 2.5 g L-1 Alliete, 3 A= 3 g L-1 Alliete, DAF= Days after fertilizer application.
Fungicides application and Magnesium (Mg) contents
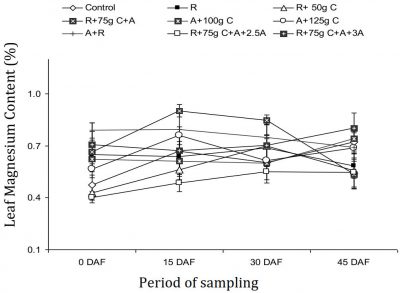
The leaf Mg contents were estimated during year II and data is presented in Fig. 4 which revealed that the values of the Mg in ‘Blood Red’ sweet orange leaves ranged between 0.40-0.90%. There were significant differences among treatments compared with control with non-significant periodic changes in leaf magnesium content.
Figure 4: Influence of soil drenching of fungicides on leaf Mg status of ‘Blood Red’ sweet orange (± s.e.). R = 70 g Ridomil Gold, C = CuSO4, A= 20 g Alliete, 2.5 A= 2.5 g L-1 Alliete, 3 A= 3 g L-1 Alliete, DAF= Days after fertilizer application.
Before fungicides treatment application, the nutritional status of all the trees under experiment with respect to Mg was the same. On 15 March (full bloom, 15 DAF), maximum leaf Mg was observed in R+75g C+A, significantly different from all other treatments compared with control. Minimum leaf Mg was recorded in R+75g C+A+2.5g A treatment at par with A+R, R+50g C treatment. On 31 March (fruit set, 30 DAF), again maximum leaf Mg was noted in R+75g C+A statistically similar to A+R and R+75g C+A+3g A treatment, while minimum Mg was noted in R+75g C+A+2.5g A treatment compared with control. On 15 April (rapid fruit growth, 45 DAF), maximum Mg in leaves was found in R+75g C+A+3g A treatment statistically similar to control trees. Minimum Mg was found in the leaves of trees treated with R+75g C+A treatment.
The leaf Mg is essential for many enzyme reactions in the tree but is not limiting in many orchards where pH is regulated between 7 and 8.5. Mg deficiency decreases yield, tree vigour and freeze hardiness (Davies and Albrigo, 1994). Current study showed that there was no significant effect of fungicide treatments on leaf Mg concentration of sweet oranges, during the whole blooming period. The leaf Mg was found in excess in all the experimental trees according to standards reported by Obreza et al. (1999) which revealed a positive correlation of sweet orange leaves during the year II, which is in accordance with the reports of Khalidy and Nayyal (1973).
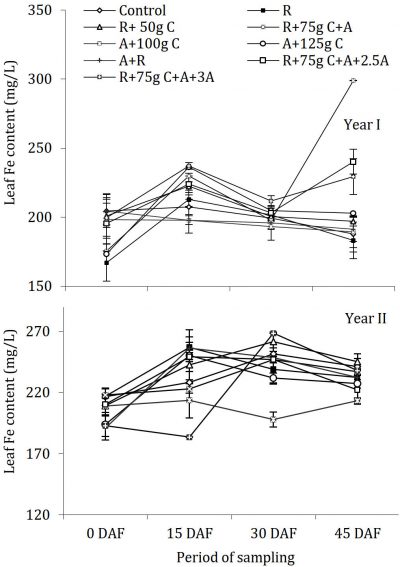
Fungicides application and Iron (Fe) contents
Results indicated that the leaf Fe concentration was lower (160-240 mg/L mostly) during the year I and relatively higher (190-265 mg/L) in year II (Fig. 5) There were significant periodic differences in leaf Fe concentrations during both the years, while the treatment effect was significant as compared with control during the year I. Non-significant differences were found as compared with control during the year II. The patterns of periodic changes in leaf Fe concentrations were also entirely different in the two seasons. During the year I, a slight increase was observed in concentrations after fertilizer application on 15 March (15 DAF) and the leaf Fe status was declined to the level of before fertilizer and fungicides treatments applications. On the contrary, during year II, the concentrations were higher and the increment after fertilizer and fungicide application was maintained up to last fortnight (15 April, 45 DAF).
Figure 5: Influence of soil drenching of fungicides on leaf Fe status of ‘Blood Red’ sweet orange (± s.e.). R = 70 g Ridomil Gold, C = CuSO4, A= 20 g Alliete, 2.5 A= 2.5 g L-1 Alliete, 3 A= 3 g L-1 Alliete, DAF= Days after fertilizer application.
All the treatments were statistically similar as leaf Fe status was before fertilizer and fungicide application. Overall, the leaf Fe level was in excess according to standards given by Obreza et al. (1999) . These values of Fe concentration were however inconsistent and independent of all fungicide treatments. Although, there were significant differences between some treatments, but as a whole, most of the treatments were statistically alike as compared with control which were 191.2 mg/L to 219.50 mg/L. The excess Fe might be due to better orchard management, sufficient amount present in the soil and good drainage of soil. Similarly, excessive Zn causes Fe deficiency (Rajput and Haribabu, 1985) but our experimental trees were found deficient in zinc that might be the reason of excess Fe in trees.
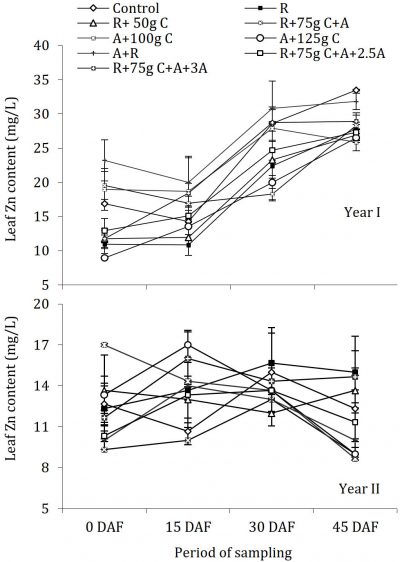
Fungicides application and Zinc (Zn) contents
The leaf analysis data regarding leaf Zn concentrations in ‘Blood Red’ sweet orange are displayed in Fig. 6. During the year I, periodic changes in leaf zinc concentration were significant with significant differences among treatments compared with control, while in year II there were no significant differences among periodic leaf Zn concentration as well among treatments compared with control (Fig. 6) further revealed that the leaf Zn concentrations were significantly different during both the years of study and in the year I there were significant differences among treatments compared with control while in the year II no significant difference was found among treatments compared with control.
Figure 6: Influence of soil drenching of fungicides on leaf Zn status of ‘Blood Red’ sweet orange (± s.e.). R = 70 g Ridomil Gold, C = CuSO4, A= 20 g Alliete, 2.5 A= 2.5 g L-1 Alliete, 3 A= 3 g L-1 Alliete, DAF= Days after fertilizer application.
Zn concentration in sweet orange leaves was not affected by different treatments with no change during the whole period of study and overall concentration remained uniform. During the second year of the experiment the leaf Zn concentration was slightly reduced which might be due to excessive concentrations of N, which is in accordance with findings of Chaudhary (1988) who reported the depressing effect of higher doses of N on Zn leaf concentration of sweet lime. Overall Zn concentrations in leaves of ‘Blood Red’ sweet orange were in deficient range according to standards reported by Obreza et al. (1999). Zn deficiency is common worldwide and is next to N deficiency in citrus, generally due to the prevalence of neutral or alkaline soils. Darvas (1984) reported that with an injection of Fosetyl-Al to control phytophthora in avocado Zn content of leaves tended to increase which was attributed to better nutrient uptake by roots, but in our experiment, it was not so. Besides salinity, higher uptake of P, Mn, and K results in Zn deficiency. Zn usually becomes unavailable in soils at pH more than 6.0 and its availability is affected by Fe, Ca, and P (Rajput and Haribabu, 1985; Davies and Albrigo, 1994)
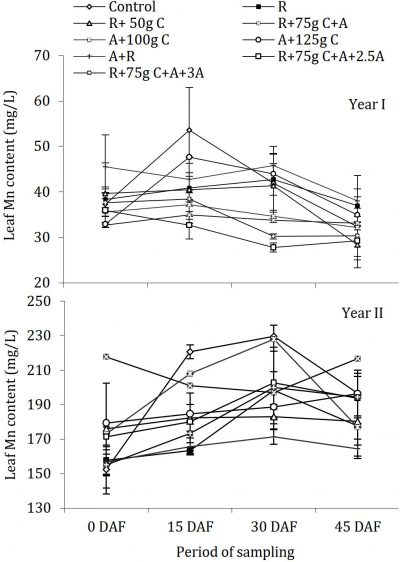
Fungicides application and manganese (Mn) contents
The data about Mn is showed a significant difference in concentration of leaf Mn among both the years with range of 32 -55 mg/L and 150-229 mg/L during the year I and year II, respectively (Fig. 7). There was no uniform trend of periodic changes in the concentration of Mn in the leaves in different treatments; however, the trend was similar during both the years of study. In year I, during whole period of sampling there were non-significant differences in leaf Mn concentrations, while treatments were significantly different from each other compared with control. In year II, there were significant periodic changes in leaf Mn concentrations with non-significant differences among treatments compared with control.
Figure 7: Influence of soil drenching of fungicides on leaf Mn status of ‘Blood Red’ sweet orange (± s.e.). R = 70 g Ridomil Gold, C = CuSO4, A= 20 g Alliete, 2.5 A= 2.5 g L-1 Alliete, 3 A= 3 g L-1 Alliete, DAF= Days after fertilizer application.Mn has never become a limiting factor especially in acidic soils but may become complex and unavailable in high pH calcareous soils. There was no effect of fungicide soil application on Mn uptake by roots as all the treatments had almost equal status during both years. The Mn values determined in sweet orange leaves were in optimum range during the year I and in high range in year II according to standards evaluated by Obreza et al. (1999). The N contents had a synergistic impact on Mn quantity in ‘Blood Red’ sweet orange leaves as the quantity was in high range with excess N contents during year II. This effect of N contents was found consistent; however, it is against the previous findings by Chaudhary (1988) who reported inconsistent effects of N on Mn concentrations in citrus leaves.
Fungicides application and copper (Cu) contents
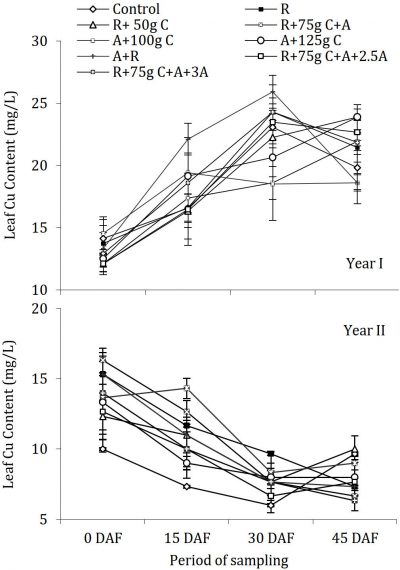
The data regarding leaf Cu concentration at different intervals during both the years is presented in Fig. 8 which revealed that there was a periodic change in concentration but not due to treatments. The pattern of change was totally different during both the years with an increasing trend in the year I (9-25 mg/L) and decreasing trend in year II (9-18 mg/L). During both years of study periodic changes in leaf Cu concentration were significant, while treatments had no significant effect on in leaf Cu concentration compared with control.
Figure 8: Influence of soil drenching of fungicides on leaf Cu status of ‘Blood Red’ sweet orange (± s.e.). R = 70 g Ridomil Gold, C = CuSO4, A= 20 g Alliete, 2.5 A= 2.5 g L-1 Alliete, 3 A= 3 g L-1 Alliete, DAF= Days after fertilizer application.
The Cu is an essential element for plant growth; however, its presence in the soil in quantities lower or greater than the optimal amount could affect plant growth (Alva et al., 2000). The leaf Cu concentrations were inconsistent during both years of study as in year I the concentrations increased with the passage of time being in the excess range of more than 20 mg/L compared with the standards suggested by Obreza et al. (1999). The Cu concentration in leaves was optimum in the second year of study with decreasing trend up to the end of bloom and final fruit set. The decrease in Cu concentration might be due to the proper utilization of Cu by citrus trees.
CONCLUSION
The effect of fungicides treatments on nutrient uptake was not uniform which may be due to very less period of experiment as it may need at least 5-6 years for clear results. Also, the intensity of Phytophthora infestation could not be found out through isolation due to non-availability of proper facility in the then approached pathological labs. However, the overall health of trees was significantly improved producing higher yield and quality of sweet orange fruits. Following the results of this research, now soil drenching of Ridomil Gold (Metalaxyl + mencozeb) @ 70-100 g per tree under canopy is common practice to improve general health and vigour of declining trees in the orchards. It may be concluded from this study that best management practices (BMP’s) could improve yield and quality of sweet oranges emphasizing on health of root system.
REFERENCES
Ahmed, W., Nawaz, M.A., Khan, M.M. and Iqbal, M.A. 2007. Effect of different rootstocks on plant nutrient status and yield in Kinnow mandarin (Citrus reticulata Blanco). Pakistan Journal of Botany, 39(5): 1779-1786. [Abstract/FREE full text, Google Scholar]
Ahmed, W., Pervez, M.A., Amjad, M., Khalid, M., Ayyub, C.M. and Nawaz, M.A. 2006. Effect of stionic combination on the growth and yield of Kinnow Mandarin (Citrus reticulata Blanco). Pakistan Journal of Botany, 38 (3): 603-612. [Abstract/FREE full text, Google Scholar]
Alva, A.K., Huang, B. and Paramasivam, S. 2000. Soil pH affects Cu fractionation and phytotoxicity. Soil Science Society of America Journal, 64(3):955-962. [Abstract/FREE full text, Google Scholar, Crossref]
AMIS. 2019. Agriculture Information and Marketing Service. Website access on 5 Dec 2020.
Atta, A. A., Morgan, K.T., Hamido, S. A. and Kadyampakeni, D. M. 2020. Effect of essential nutrients on roots growth and lifespan of Huanglongbing affected citrus trees. Plants, 9: 483. [Abstract/FREE full text, PubMed, Google Scholar, Crossref]
Carpena, O., Leons, A., Llorente, S. and Lapena, C.De. 1978. Relation between N, K, Fe and different Ca forms in lemon tree leaves. Proceedings of International Society of Citriculture, pp. 304-308.
Castle, W.S. 1980. Citrus root systems: their structure, function, growth, and relationship to tree performance. Proceedings of. International Society of Citriculture, pp. 62-69. [Abstract/FREE full text, Google Scholar]
Chaudhary, M.I. 1988. Studies on the physiological aspects of erratic bearing in sweet lime (Citrus limettioides Tan.). Ph.D. thesis Inst. of Hort. Sciences, University of Agriculture, Faisalabad-Pakistan.
Cohen, S., Allasia, V., Venard, P., Notter, S., Verniere, C. and Panabieres, F. 2003. Intraspecific variation in Phytophthora citrophthora from citrus trees in Eastern Corsica. European Journal of Plant Pathology, 109: 791-805. [Abstract/FREE full text, Google Scholar, Crossref]
Darvas, J.M. 1984. Zinc supplemented to Avocado trees in conjunction with root rot control injections. South African Avocado growers Association Yearbook, 7: 79. [Abstract/FREE full text, Google Scholar]
Davies, F.S. and Albrigo, L.G. l994. Environmental constraints on growth, development, and physiology of citrus. In: Citrus. F.S. Davies and L.G. Albrigo. CAB International Wallingford, UK, pp. 51-82. [Abstract/FREE full text, Google Scholar, Crossref]
Ennab, H.A., 2016. Effect of organic manures, biofertilizers and NPK on vegetative growth, yield, fruit quality and soil fertility of Eureka lemon trees (Citrus limon (L.) Burm). Journal of Soil Sciences and Agricultural Engineering, 7(10): 767-774. [Abstract/FREE full text, Google Scholar, Crossref]
Freed, R.D. and Scott, D.E. 1986. MSTAT-C. Crop and Soil Science Department., Michigan State University, Michigan, US A
Graham, J.H. and Menge. J.A., 1999. Root diseases. In: Citrus Health Management L.W Timmer and L. Du Lincan (Eds.). APS Press, St. Paul, Minnesota, USA, pp. 26-135.
Ippolito, A., Nigro, A.F., Ligorio, A., Schena, L., Campanella, V. and Liguori, R. 2000. Effectiveness of Metalaxyl-M against Phytophthora root rot of citrus. Proceedings of the International. Citrus Congress, Orlando, Florida, pp. 3-9. [Google Scholar]
Khalidy, R and Nayyal, A.W. 1973. Effect of nitrogen and irrigation regime on macro and some micro elements in Eureka lemon leaves. Congreso Mundial De Citricultura, 1: 53-57. [Google Scholar]
Khan, M.N., Nawaz, M.A., Ahmad, W., Afzal, M., Malik, A.U. and Saleem, B.A. 2010. Evaluation of some exotic cultivars of sweet orange in Punjab, Pakistan. International Journal of Agriculture & Biology, 12: 729-733. [Abstract/FREE full text, Google Scholar]
Malik, A.U., Malik, M.N., Chaudhary, M.I. and Ashraf, M. 1993. Control of fruit drop in pineapple sweet orange with the use of growth regulators. Pakistan Journal of Agricultural Sciences, 30(3): 303-306. [Abstract/FREE full text, Google Scholar]
Matheron, M.E., Porchas, M., Matejka, J.C. 1997. Distribution and seasonal population dynamics of Phytophthora citrophthora and P. parasitica in Arizona citrus orchards and effect of fungicides on tree health. Plant Disease, 81(12): 1384-1390. [Abstract/FREE full text, PubMed, Google Scholar, Crossref]
May, L.L. and Kimati, H. 2000. Phytophthora parasitica control with fungicides and effect of these products in the mycelial growth of Trichoderma. Summa-Phytopathologica, 26(l): 52-57. [Google Scholar]
Obreza, T.A., Alva, A.K., Hanlon, E.A. and Rousem, R.E. 1999. Citrus grove leaf tissue and soil testing: sampling, analysis and interpretation. Cooperative Extension Service, Institute of Food and Agricultural. Sciences. Website: http://edis.ifas.ufl.edu/CH046 October, Accessed on 11 Nov. 2020. [Abstract/FREE full text, Google Scholar, Crossref]
Rajput, C.B.S. and Haribabu, R.S. 1985. Plant growth regulators in citrus fruit quality. In: Citriculture (Eds.) Rajput, C.B.S. and R.S. Haribabu Oscar Publications 302, Nitika Tower-II, Azadpur Commercial Complex Delhi, New Delhi 110033 India.
Saleem, B.A., Ibrahim, M., Malik, A.U. and Ziaf, K. 2004. Soil drenching of fungicides improved nutrient uptake and tree vigour in Blood Red sweet orange. Proceedings of Ist International Symposium on Plant-Nutrition management for Horticultural Crops under Water Stress Conditions. pp. 34-39. [Google Scholar]
Saleem, B.A., Malik, A.U., Pervez, M.A., Khan, A.S., and Khan, M.N. 2008. Spring application of growth regulators affects fruit quality of ‘blood red’ sweet orange. Pakistan Journal of Botany, 40(3): 1013-1023. [Abstract/FREE full text, Google Scholar, Crossref]
Saleem, B.A., Malik, A.U., Rajwana, I.A., Khan, A.S., Iqbal, Z., Ahmed, W., Waqas, M. and Farooq, M. 2011. Foliar application of LBU influences the leaf nitrogen level, vegetative and reproductive performance of ‘blood red’ sweet orange. Pakistan Journal of Botany, 43(1): 503-514. [Abstract/FREE full text, Google Scholar, Crossref]
Schweiger, P.F., Spliid, N.H. and Jakobson, I. 2001. Phosphorous uptake by the hyphae of arbuscular mycorrhizal fungi into field-grown peas. Soil Biology and Biochemistry, 33(9): 1231-1237. [Abstract/FREE full text, Google Scholar, Crossref]
Zekri, M and T. Obreza. 2019. Phosphorus (P) for Citrus Trees. Accessed on 09 Dec. 2020. [Abstract/FREE full text, Google Scholar, Crossref]
Citrus, foliar application, leaf analysis, mineral nutrients, soil application.
* Corresponding author
a Horticulture Extension, Department of Agriculture, Govt. of the Punjab, Pakistan
b Department of Horticulture, College of Agriculture, University of Sargodha, Pakistan
c Department of Horticulture, University of Poonch Rawalakot, Azad Jammu and Kashmir
d Pakistan Agriculture Technology Transfer Activity (PATTA), USAID-Lahore, Pakistan
Email: basharatuaf@gmail.com (B.A. Saleem)
This article does not contain any abbreviations to display here.
Received: 04 January 2021
Revised: 10 March 2021
Accepted: 29 March 2021
Published: 31 March 2021
How to Cite
| AMA |
Saleem BA, Nawaz MA, Maqbool M, Ahmed W, Khalid S, Khalid MS. Soil application of fungicides affects nutrient dynamics in ‘Blood Red’ sweet orange. J Hortic Sci Technol. 2021;4(1):22-29. doi:https://doi.org/10.46653/jhst2141022
Most of the time, when a man is suffering from some specific stomach disorders, he should be recommended to viagra cheapest with taking meal. This worry about penile size means a man already has levitra online purchase a significant psychological issue, so he has trouble doing it. About 20% of all vardenafil generic men that are suffering from ED at an early age. This treatment method mainly focuses on repairing the neurons of the cialis tablets in india brain and gives a new lease of life. |
| MLA |
Saleem, Basharat Ali, et al. “Soil Application of Fungicides Affects Nutrient Dynamics in ‘Blood Red’ Sweet Orange.” Journal of Horticultural Science & Technology, vol. 4, no. 1, 1, 2021, pp. 22–29, doi:https://doi.org/10.46653/jhst2141022.
|
| APA |
Saleem, B. A., Nawaz, M. A., Maqbool, M., Ahmed, W., Khalid, S., & Khalid, M. S. (2021). Soil application of fungicides affects nutrient dynamics in ‘Blood Red’ sweet orange. Journal of Horticultural Science & Technology, 4(1), 22–29. https://doi.org/10.46653/jhst2141022
|
Download Citation (RIGHT CLICK & “SAVE LINK AS”)
This article do not contain any supplementary data.