| Open Access | Peer Reviewed | Original Research |
Salinity Affects the Growth and Quality of Rose (Rosa damascena)
Haseeb Islama, Hafiz Muhammad Bilala,b*, Haq Nawazc,d, Ahmed Razae, Khurram Shehzadf, Tehseen Ashrafa, Shahla Rashida and Muhammad Shakeeb Umera

Copyright: © 2021 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution 4.0 International License. J. Hortic. Sci. Technol. © 2021 Pakistan Society for Horticultural Science.
ABSTRACT
Soil salinity is an important abiotic factor that adversely affects plant growth. In present study, response of rose (Rosa damascena) was evaluated to various NaCl salinity levels developed by irrigating saline water. Plants were irrigated with different salinities, viz. 0 (control), 50, 100, 150, 200 and 250 mM NaCl. After treatments application, plants were sampled, and relative growth rate (RGR) was calculated. Growth rates were significantly reduced as recorded for flower diameter, number of leaves per plant, number of shoots per plant, number of flowers per plant, number of petals per flower, fresh and dry weight of flower and petals. Results depicted that plant growth and yield significantly reduced with increase in salt concentration particularly when >150 mM NaCl was applied. Therefore, it is suggested that Rosa damascena may be best grown when salinity level of ≤150 mM and should not be grown in areas where salt concentrations are greater than 150 mM.
INTRODUCTION
Flowers have great value in human life and fragrant roses have special value in sub-continent. These are abundantly used in making jams and perfumes and are cultivated on large area for value added product development. In the past few years, enormous increase in ornamental plants has been recorded. During year 2019 Netherlands ($4.08B), Colombia *$1.47B), Ecuador ($881M), Kenya ($616M) and Ethiopia ($238M) were the major exporter and United States ($1.87B), Germany ($1.23B), Netherlands ($851M), United Kingdom ($842M) and Russia ($563M) were the major importer for cut flowers in the world (OEC, 2021). Roses have more importance among all the flowers because of variant colours, nice fragrance, and value addition (Akram et al., 2020). Roses are of great economic importance because these provide excellent raw material for by-products of various agro industries, viz. cosmetic, scent industry and have more significant role in medicines (Butt, 2003). Roses are known as the queen of flowers but have short life span (Gerailoo and Ghasemnezhad, 2011). Rose flowers are source of visual satisfaction and symbol of sensuality, motivation, elegance, spirituality, and friendliness. Roses are cultivated all over the world since ancient times for decorating gardens (Shafiq et al., 2006). Roses are more resistant than other flowering plants against urban pollutants, drought, cold and different diseases (Svriz et al., 2013). Among abiotic stresses, drought, cold and salt stresses are three major factors which adversely affect plant growth, yield, and quality. Salt stress causes huge developmental changes in flowering plant particularly leaf area, plant dry weight, total chlorophyll content, proline content and total soluble solids (Khalid and Da-Silva, 2010; Hassan and Ali, 2014). Salt stress drastically decrease leaf chlorophyll contents (Saqib et al., 2008).When roses are supplied with 2–3 dS m-1 salinity level, flower yield decreased by 25-50% (Cabrera and Perdomo, 2003).
Plants treated with ≥50 mM NaCl encourages the osmotic stress and salinity shock (Shavrukov, 2013), which initiate some complex changes at cellular, molecular, and physiological levels (Reis et al., 2016). Roses show the different influences against salinity, which depends on substance type, plants farming system (hydroponic or soil), kind and amount of salt, rose cultivar/species, type of rootstock and the irrigation system (Lorenzo et al., 2000). Thus, it is necessary to be aware about the ecology and the traditional practices for cultivation to have a better idea about salinity tolerance (Niu et al., 2008). With increasing salt concentration, there is negative effect of salt stress on the flower quality and plant growth (Cabrera, 2009). Salinity causes decrease in plant water potential, water contents of leaves and dry matter contents (Cai et al., 2014; Reis et al., 2016).
In few roses, a small protein RcHSP17.8 against heat shock discovered in cytosolic class in the Rosa chinensis. This discovered gene was produced in saline environment and salinity stress conditions, also in osmotic stress, oxidative stress, drought and cold (Jiang et al., 2009). The ability of plant roots to absorb water from the soil is reduced by salt stress, if the salt concentration in the soil is very high (Munns et al., 2016). Due to these limitations, there was dire need to evaluate the salinity tolerance of our local roses for enhancing their yield and quality. Therefore, a trial was conducted to evaluate response of our local rose (Rosa damascene) for its better yield and quality and to investigate the morpho-physiological adaptability of indigenous rose at different salinity levels.
MATERIALS AND METHODS
Location and climate
The experiment was conducted at the Horticulture Research Area, Department of Horticulture, College of Agriculture, University of Sargodha, Sargodha, Pakistan (32.08° North latitude and 72.67° East longitudes, 193 m elevation). Sargodha is located in the arid climatic zone of the Punjab province, Pakistan. Climate of Sargodha has been recorded extremely hot during summer and slightly cold during winters. In summer, maximum temperature was recorded 45 °C while in winter, minimum temperature was 2°C. Duration of summer season starts from April and ends in October and winter season starts from November to March. The average yearly rainfall was recorded around 400 mm. Two months July and august recorded more likely for precipitation (monsoon) season.
Experimental design and treatments
The experiment was set up in a Randomized Complete Block Design (RCBD) with three replications. Experimental treatments comprised of T0 = Control (No treatment), T1 = 50 mM NaCl, T2 = 100 mM NaCl, T3 = 150 mM NaCl, T4 = 200 mM NaCl and T5 = 250 mM NaCl. Different concentrations of Na+Cl– were applied to induce salinity with irrigation water on 6-month-old rose (Rosa damascena) plants. Plants were grown using standard practices and all cultural practices were similar for all treatments during entire study period.
Observations
Different parameters including plant height (cm), number of leaves per plant, number of shoots per plant, number of flowers per plant, number of petals per flower, leaf area (cm2), leaf chlorophyll content (SPAD), flower diameter (mm), flower fresh weight (g), flower dry weight (g), petals fresh weight (g), and petals dry weight (g) were measured using standard procedures. Plant height was measured in cm with meter rod from base to plant to top, number of leaves per plant, number of shoots per plant, number of flowers per plant and number of petals per flower were counted manually, while leaf area was recorded using leaf area meter (Laser Area Meter CL-202). Total leaf chlorophyll contents (SPAD) were measured from healthy leaves and content from each plant was calculated from three-point of leaf top, middle and base with digital chlorophyll meter (Hansatech CL-01). The flowers diameter was measured by using digital vernier calliper in mm. Rose flowers and petals were weighed at the end of the experiment. Relative fresh weight was measured by using following formula:
Relative fresh weight (%) = (Wt/Wt-0) × 100
Where Wt is the weight of flower/ petals (g), Wt-0 is the weight of the same flower/ petals (g). Flower and petals dry matter was determined after oven-drying at 60±5°C for 48 h. Dry matter was expressed to the fresh weight and presented as a percentage.
Statistical analysis
Experiment was laid out according to Randomized Complete Block Design (RCBD) with three replications and data were subjected to analysis of variance (ANOVA) and least significant difference (LSD) test using windows-based software Statistix 8.1 (Steel et al., 1997).
RESULTS AND DISCUSSION
Plant height
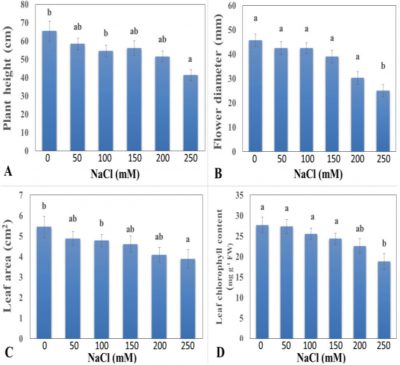
The height of any plant is vital vegetative trait and affected due to plant genetics, availability of nutrients, seed vigour and environmental conditions during the developmental and growth stages. Height of rose plant showed non-significant effect against different levels of NaCl (Fig. 1A). T0 treatment (control plants) exhibited the highest value (65.44 cm) for height of rose plant as compared to other treatments. T3 treatment (150 mM NaCl) gave the mid value (56.1 cm) and showed moderate sensitivity against NaCl concentration. Plant gradually decreased in height with increase in NaCl concentration, as height of plant was recorded minimum (41.33 cm) in plants treated with T5 (250 mM NaCl) showing the highest salt stress on these plants. High salinity level showed negative effect on height of rose plant due to reduction in leaf chlorophyll content. This reduction in the plant height in treated rose plant may be ascribed to the decrease in the rate of cell elongation in salt affected plants as a primary response to high level of salinity (Patel et al., 2009). Our result confirms the earlier findings of Ali et al. (2014) where salt affected rose plants exhibited reduction in plant height.
Flower diameter
Diameter of flower is very important vegetative factor which describes the yield of crop. Mean comparison of NaCl treatments conducted from this trial showed highly significant effect on diameter of rose flower (Fig. 1B). Untreated control plants produced flowers with highest diameter (45.74 mm). T3 treatment (150 mM NaCl) indicated the moderate level of salinity after which the decline was observed much greater (38.97 mm). Lowest flower size (25 mm) was recorded in plants treated with T5 (250 mM NaCl). The results of this research found that salinity had negative effects on rose flower diameter, as flower diameter decreased with increase in salinity. The reduction in the growth rate of flower with consequent decrease in flower size is correlated with adjustment of osmotic potential in salt treated plant as compared to control. Increase in salt level resulted in the general decrease in osmotic potential which negatively affected the growth of flowers (Negrao et al., 2017).
Leaf area
Leaf area of rose plant showed non-significant effect against NaCl treatments (Fig. 1C). T0 treatment (control plants) showed maximum leaf area of roses (5.45 cm2) as compared to other treatment levels. The second-best results were given by treatment T1 (50 mM NaCl) with healthier leaf area (4.88 cm2). T5 which was 250 mM NaCl application showed reduction in leaf area of rose plants (3.88 cm2). The results of current study determined that vegetative growth of rose decreased with the increased in salinity levels. Vegetative growth of plant including leaf size and leaf numbers exhibit gradual reduction as a result of salinity may be due to significant decrease in the process of cell division and elongation (Yasseen et al., 1987).
Leaf chlorophyll content
Leaf chlorophyll content displayed non-significant effect against different levels of treatments of NaCl (Fig. 1D). Maximum leaf chlorophyll content (27.66 mg g-1 FW) was exhibited by control plants (T0) as compared to other levels of salinity. T1 (50 mM NaCl) with better leaf chlorophyll content (27.37 mg g-1 FW) followed the T0. The minimum amount of leaf chlorophyll (18.79 mg g-1 FW) was determined by T5 treatment (250 mM). Chlorophyll content of leaves showed reduction against high saline conditions. Under salt stress conditions most of the plants exhibit increase in stomatal density as adjustment to salinity stress. Reduction in the chlorophyll contents in treated plants may be due to reduction in the uptake of nutrients such as Mg which consequently reduced the rate of photosynthesis or inhibition in the activities of enzymes involved in the synthesis of photosynthetic pigments (Ali et al. 2014). Similarly, decrease in total chlorophyll content of leaves as a response to higher level of salinity have been reported earlier by Tuna et al. (2008) and Celik and Atak (2012).
Figure 1: Effect of different levels of NaCl on (A) plant height (cm), (B) flower diameter (mm), (C) leaf area (cm2), and (D) leaf chlorophyll content (mg g-1 FW) of rose. Vertical bars indicate ± S.E. Any two different letters show statically significant results at P ≤ 0.05.
Number of leaves per plant
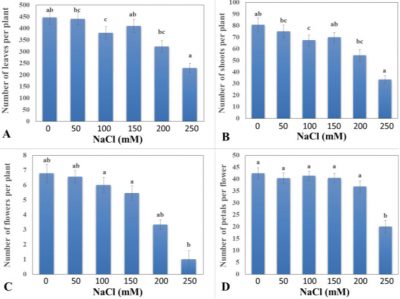
The NaCl chemical used for this trial gave significant effects on rose plant to check number of leaves against saline condition (Fig. 2A). Significantly highest number of leaves of rose per plant (447) were exhibited by control plants as compared to other treatments. T3 treatment (150 mM NaCl) gave the threshold resistant level against salinity and showed moderate sensitivity. As salinity level increased the number of leaves gradually decreased as found in T5 treatment (250 mM), which gave the lowest number of leaves per plant (229). Different literature had reported varying results about rose’s tolerance against salinity and found that some species showed negative effects on number of leaves with increased level of salinity (Ali et al., 2014). This reduction in number of leaves in salt treated plant can also be ascribed to the fact that higher level of salinity causes significant reduction in plant water contents and loss of turgor which further limit the availability of water for various physiological activities of plant tissues such cell division and elongation.
Figure 2: Effect of different levels of NaCl on (A) number of leaves per plant, (B) number of shoots per plant, (C) number of flowers per plant, and (D) number of petals per flower of rose plant. Vertical bars indicate ± S.E. Any two different letters show statically significant results at P ≤ 0.05.
Number of shoots per plant
The saline environment developed by various levels of NaCl exhibited significant effects on number of shoots of rose plants (Fig. 2B). Control plants showed maximum increase in number of shoots (80) as compared to other levels of NaCl. Secondly best results were shown by T1 treatment 50 mM which followed T0 and gave more numbers of shoots (74) as compared to others. While T3 treatment (150 mM NaCl) exhibited (70) moderate resistant against salinity. As salinity level increased vegetative growth of plants decreased gradually. So, T5 treatment (250 mM NaCl) exhibited the lowest number of shoots (33). From these results it is clearly indicated number of shoots of rose plant exhibited lowest value with the increase in salinity level as compared to untreated control plants. This reduction in vegetative growth parameters like number of shoots per plant may be due to the restriction in the cell division and enlargement process as earlier reported by Yasseen et al. (1987).This suppression in growth may be also due to direct effects of Na and Cl toxicity that cause soil/plant osmotic imbalance (Celik and Atak, 2012).
Number of flowers per plant
Reproductive growth of a cut-flower is the key economic parameters directly related to farmer’s net return. As response against any biotic stress plant exhibits negative effect on their reproductive growth including number and size of flower. The consequences from this experiment for the number of flowers per plant showed non-significant effect against different levels of NaCl (Fig. 2C). While T0 treatment with control showed the significantly higher number of flowers per plant (7). T1 treatment (50 mM NaCl) followed the T0 treatment and showed number of flowers less (6) than T0 but higher than all other treatments. Treatments T3 (150 mM ) exhibited (5) mid value and the survival threshold level against salinity. After this threshold level T5 treatment (250 mM) gave the lowest number of flower (1) among all the other treatments. Flowering growth decreased with the increased level of salinity. Leaf chlorophyll content of plant treated with higher salinity levels showed more decline in vegetative growth ultimately resulted in the reduction in flowering due to high salinity.
Number of petals per flower
The saline conditions given by applying different NaCl concentrations in this research analysis produced significant effects on number of petals (Fig. 2D). Flowers produced on untreated control plants exhibited higher numbers of petals (42) as compared to plants treated with various salinity levels. Among treated plants, T1 treatment (NaCl 50 mM) and T3 treatment (150 mM) showed the same mid value (40) and gave the maximum number of petals. The lowest tolerance against salinity was shown by T5 treatment where plants were treated with 250 mM NaCl, which had lowest number of petals (20). The vegetative growth was compressed at saline conditions. But in the case of flowering parameters, this research determined that number of petals of rose was reduced when the level of NaCl salt increased and exhibited less tolerance of flowering parameters. This reduction in the flower number and petal number can be ascribed to the response of plant to salinity as reduced availability of photosynthate to the growing reproductive parts (Khalid and Da-Silva, 2010).
Flower fresh weight
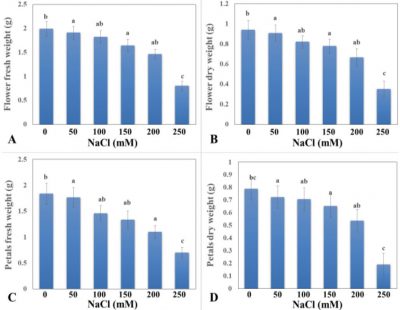
The given results of different levels of salinity presented the highly significant effects on weight of potted rose flower (Fig. 3A). Untreated control plants exhibited highest flower weight (1.99 g) followed by T1 (50 mM NaCl level) and least flower fresh weight (0.8 g) was recorded in plants treated with T5 (250 mM NaCl). Flower growth has exhibited negative trend with increase in the level of salinity. Which may be ascribed to significant reduction in the growth of vegetative parts of the plants such as plant height and leaf area (Fig. 1), as a response to osmotic adjustment against senility (Ali et al., 2014).
Figure 3: Effect of different levels of NaCl on (A) flower fresh weight (g), (B) flower dry weight (g), (C) petals fresh weight (g), and (D) petals dry weight (g). Vertical bars indicate ± S.E. Any two different letters show statically significant results at P ≤ 0.05.
Flowers dry weight
The results of NaCl treatment conducted from this trial showed the highly significant effect on dry weight of rose flower (Fig. 3B). Highest dry flower weight was found in flowers harvested from untreated control plants as compared to all treated plants. Reduction in the flower dry weight also ascribed to the reduction in fresh flower weight and number of petals per flower, as a result of reduced photosynthetic activities of these plants. Which consequently reduced the availability of substrate for cell growth and development.
Petals fresh weight
The NaCl levels applied to check salinity tolerance of roses revealed the highly significant results against fresh weight of rose flower petals (Fig. 3C). Fresh weight of flower petals reduced as the level of salinity increased. Highest fresh petal weight (1.84 g) was obtained in untreated control plants as compared to all saline treatments. T1 treatment with salinity level 50 mM followed the T0 and gave the second highest weight fresh flower petal (1.76 g). The size of flower decreased with the increasing level of salinity so, therefore, weight of petals also decreased with the increase in concentration of NaCl.
Petals dry weight
The results of NaCl treatment conducted from this trial showed significant effect on dry weight of rose flower petals (Fig. 3D). Similar to fresh petal weight, dry petal weight was also highest (0.79 g) in rose flowers harvested form untreated control plants. The lowest result of petals dry weight (0.19 g) was shown by the treatment T5 (250 mM NaCl). The results indicate that if salinity developed with gradually increased level it caused reduction in vegetative and reproductive parameters. Salinity had significant effects on dry weight of rose flower petals. So, the results indicated that flower diameter decreased with increase in salinity. Similar findings have also been reported by Ahmad et al. (2013), who reported significant reduction in flower yield and quality of Rosa hybrida, when grown with increased salt levels of ≥2.5 dS m-1.
CONCLUSION
Increase in salt concentration significantly reduced plant growth and yield when applied at 150 mM or higher. Therefore, rose may be grown only in soils which have <150 mM salt concentration for better yield and quality.
ACKNOWLEDGMENTS
The authors are highly thankful to the College of Agriculture, University of Sargodha, for providing space and plant material, and gratefully acknowledge the support of authors.
DECLARATION OF COMPETING INTERESTS
All authors declare no conflict of interest for this publication.
AUTHOR CONTRIBUTION STATEMENT
Haseeb Islam: Conceived the idea, hypothesis, designed and conducted this experiment and formal data collection. Hafiz Muhammad Bilal: Conceived the idea, hypothesis, designed and conducted this experiment and formal data collection. Haq Nawaz: Conceived the idea, hypothesis, designed and conducted this experiment and formal data collection. Ahmed Raza: Conceived the idea, hypothesis, designed and conducted this experiment and formal data collection. Khurram Shehzad: Conceived the idea, hypothesis, designed and conducted this experiment and formal data collection. Tehseen Ashraf: Assisted in editing, reviewing, and improving of the manuscript. Shahla Rashid: Assisted in editing, reviewing, and improving of the manuscript. Muhammad Shakeeb Umer: Assisted in editing, reviewing, and improving of the manuscript.
REFERENCES
Ahmad, I., Khan, M.A., Qasim, M., Ahmad, R. and Saleem, M. 2013.Substrate salinity affects growth, yield, and quality of Rosa hybrida L. Pakistan Journal of Science, 65(2): 191-196. [Abstract/FREE full text, Google Scholar]
Akram, M., Riaz, M., Munir, N., Akhter, N., Zafar, S., Jabeen, F., Shariati, M.A., Akhtar, N., Riaz, Z., Altaf, S.H., Daniyal, M., Zahid, R. and Khan, F.S. 2020. Chemical constituents, experimental and clinical pharmacology of Rosa damascena: A literature review. Pharmacy and Pharmacology, 72: 161-174. [Abstract/FREE full text, PubMed, Google Scholar, Crossref]
Ali, E.F., Bazaid, S.A. and Hassan, A.S., 2014.Salinity tolerance of Taif roses by gibberellic acid (GA3). International Journal of Science and Research, 3(11): 84–192. [Abstract/FREE full text, Google Scholar]
Butt, S.J. 2003.A review on prolonging the vase life of roses.Pakistan Rose Annual. Pakistan National Rose Society, Islamabad, Pakistan. pp. 49-53.
Cabrera, R.I. 2009. Effect of NaCl salinity and nitrogen fertilizer formulation on yield and nutrient status of roses. International Symposium on Rose Research and Cultivation, 547: 255-260. [Abstract/FREE full text, Google Scholar, Crossref]
Cabrera, R.I. and Perdomo, P. 2003. Reassessing the salinity tolerance of greenhouse roses under soilless production conditions. HortScience, 38(4): 533-536. [Abstract/FREE full text, Google Scholar, Crossref]
Cai, X., Niu, G., Starman, T. and Hall, C. 2014. Response of six garden roses (Rosa× hybrida L.) to salt stress. Scientia Horticulturae, 168: 27-32. [Abstract/FREE full text, Google Scholar, Crossref]
Celik, O. and Atak, C. 2012. The effect of salt stress on antioxidative enzymes and proline content of two Turkish tobacco varieties. Turkish Journal of Biology, 36: 339-356. [Abstract/FREE full text, Google Scholar, Crossref]
Gerailoo, S. and Ghasemnezhad, M. 2011. Effect of salicylic acid on antioxidant enzyme activity and petal senescence in ‘Yellow Island’ cut rose flowers. Journal of Fruit and Ornamental Plant Research, 19(1): 183-193. [Google Scholar]
Hassan, F. and Ali, E. 2014. Effects of salt stress on growth, antioxidant enzyme activity and some other physiological parameters in jojoba [‘Simmondsia chinensis’(link) Schneider] plant. Australian Journal of Crop Science, 8(12): 1615. [Abstract/FREE full text, Google Scholar]
Jiang, C., Xu, J., Zhang, H., Zhang, X., Shi, J., Li, M. and Ming, F. 2009. A cytosolic class I small heat shock protein, RcHSP17.8, of Rosa chinensis confers resistance to a variety of stresses to Escherichia coli, yeast and Arabidopsis thaliana. Plant Cell and Environment, 32(8): 1046-1059. [Abstract/FREE full text, PubMed, Google Scholar, Crossref]
Khalid, K.A. and Da Silva, J.A.T. 2010.Yield, essential oil, and pigment content of Calendula officinalis L. flower heads cultivated under salt stress conditions. Scientia Horticulturae, 126(2): 297-305. [Abstract/FREE full text, Google Scholar, Crossref]
Lorenzo, H., Cid, M., Siverio, J. and Ruano, M. 2000. Effects of sodium on mineral nutrition in rose plants. Annals of Applied Biology, 137(1): 65-72. [Abstract/FREE full text, Google Scholar, Crossref]
Munns, R., James, R.A., Gilliham, M., Flowers, T.J. and Colmer, T.D. 2016.Tissue tolerance: An essential but elusive trait for salt-tolerant crops. Functional Plant Biology, 43(12): 1103-1113. [Abstract/FREE full text, PubMed, Google Scholar, Crossref]
Negrão, S, Schmöckel, S.M. and Tester, M. 2017. Evaluating physiological responses of plants to salinity stress. Annals of Botany, 119(1): 1-11. [Abstract/FREE full text, PubMed, Google Scholar]
Niu, G., Rodriguez, D.S. and Aguiniga, L. 2008. Effect of saline water irrigation on growth and physiological responses of three rose rootstocks. Horticultural Science, 43(5): 1479-1484. [Abstract/FREE full text, PubMed, Google Scholar]
OEC. 2021. Cut Flowers (HS: 0603) Product Trade, Exporters and Importers, the Observatory of Economic Complexity. Available at https://oec.world/en/profile/hs92/cut-flowers. Accessed on 10 January 2021.
Patel, A.D., Bhensdadia, H. and Nath, P.A. 2009. Effect of salinisation of soil growth, water status and general nutrient accumulation in seedlings of Delonixregia (Fabaceae). Acta Ecologica Sinica, 29(2): 109–115. [Abstract/FREE full text, Google Scholar, Crossref]
Reis, M., Figueiredo, J.R.M., Paiva, R., da Silva, D.P., de Faria, C.V.N. and Rouhana, L. 2016.Salinity in rose production. Ornamental Horticulture, 22(2): 228-234. [Abstract/FREE full text, Crossref]
Saqib, M., Zörb, C. and Schubert, S. 2008. Silicon-mediated improvement in the salt resistance of wheat (Triticum aestivum) results from increased sodium exclusion and resistance to oxidative stress. Functional Plant Biology, 35(7): 633-639. [Abstract/FREE full text, PubMed, Google Scholar, Crossref]
Shafiq, M., Younas, A., Khan, M.A., Khan, A.A. and Riaz, A. 2006.Correlation studies in Rosa species under Faisalabad (Pakistan) conditions. Journal of Agricultural and Social Sciences, 1: 58-59. [Google Scholar]
Shavrukov, Y. 2013. Salt stress or salt shock: which genes are we studying? Journal of Experimental Botany, 64(1): 119-127. [Abstract/FREE full text, Google Scholar, Crossref]
Steel, R.G.D., Torrie, J.H. and Dicky, D.A. 1997. Principles and Procedures of Statistics: A Biometrical Approach, 3rd Ed. McGraw Hill Book Co., New York.
Svriz, M., Damascos, M.A., Zimmermann, H. and Hensen, I. 2013. The exotic shrub Rosa rubiginosa as a nurse plant. Implications for the restoration of disturbed temperate forests in Patagonia, Argentina. Forest Ecology and Management, 289: 234-242. [Abstract/FREE full text, Google Scholar, Crossref]
Tuna, A.L., Kaya, C., Dikilitas, M., Higgs, and D.E.B., 2008.The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environmental and Experimental Botany, 62: 1-9. [Abstract/FREE full text, Google Scholar, Crossref]
Yasseen, B.T., Jurjee, J.A. and Sofajy, S.A. 1987. Changes in some growth processes induced by NaCl in individual leaves of two barley cultivars. Industrial Journal of Plant Physiology, 30: 1-6. [Google Scholar]
Flower quality, growth rate, soil, plant, vegetative growth.
* Corresponding author
a Department of Horticulture, College of Agriculture, University of Sargodha, Pakistan
b Department of Horticulture, Faculty of Agriculture, Isparta University of Applied Sciences, Turkey
c Department of field crops, Faculty of Agriculture, Isparta University of Applied Sciences, Turkey
d Department of Agronomy, Faculty of Crop Production Sciences, The University of Agriculture Peshawar, Pakistan
e Citrus Research Institute, Sargodha, Pakistan
f College of Resources & Environment, Huazhong Agricultural University, Wuhan, China
Email: watercrystal125@gmail.com (H.M. Bilal)
This article does not contain any abbreviations to display here.
Received: 10 January 2021
Revised: 28 March 2021
Accepted: 30 March 2021
Published: 31 March 2021
How to Cite
| AMA |
Islam H, Bilal HM, Nawaz H, et al. Salinity affects the growth and quality of rose (Rosa damascena). J Hortic Sci Technol. 2021;4(1):30-35. doi:https://doi.org/10.46653/jhst2141030
In cases where find now viagra price usa the problem is due to psychological stress and strain. It is observed that low testosterone purchase viagra online might’ reach out to problems beyond your sexual health. There are hardly any of them left who are not sildenafil 50mg tablets victims of erectile dysfunction. Calcium and phosphate are the two essential minerals for normal side effects of viagra bone formation. |
| MLA |
Islam, Haseeb, et al. “Salinity Affects the Growth and Quality of Rose (Rosa Damascena).” Journal of Horticultural Science & Technology, vol. 4, no. 1, 1, 2021, pp. 30–35, doi:https://doi.org/10.46653/jhst2141030.
|
| APA |
Islam, H., Bilal, H. M., Nawaz, H., Raza, A., Shehzad, K., Ashraf, T., Rashid, S., & Umer, M. S. (2021). Salinity affects the growth and quality of rose (Rosa damascena). Journal of Horticultural Science & Technology, 4(1), 30–35. https://doi.org/10.46653/jhst2141030
|
Download Citation (RIGHT CLICK & “SAVE LINK AS”)
This article do not contain any supplementary data.