Copyright: © 2021 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution 4.0 International License. J. Hortic. Sci. Technol. © 2021 Pakistan Society for Horticultural Science.
ABSTRACT
Jamun [Syzygium cumini (L.)] is a tropical evergreen tree and its scrumptious fruit is consumed as fresh and processed. It is famous for its delicious taste and aroma, use for medicinal purposes. It is a rich source of antioxidants, vitamins, phenolic contents, minerals, and edible oils that are helpful for consumers’ health. There is negligible information about cultivars due to the lack of genetic analysis of available clones. The tree can withstand water scarcity, the fruit is highly perishable with a limited shelf life of 3 days under ambient conditions and its marketing is confined to the local markets. Cold storage has been used to enhance its marketing window after pre-treatments by anti-ripening chemicals, edible coating, and non-chemical processing. In addition, modified atmosphere packaging (MAP) has also been found effective to increase its shelf life by 30 days. Nevertheless, limited research has been conducted to increase its postharvest life. This review includes preharvest factors affecting postharvest physiology, biochemical changes during fruit ripening, harvest maturity and postharvest techniques to extend shelf life and postharvest storability. This article provides the way forward for further R&D work concerning shelf-life extension and strengthens the Jamun industry with sustainable solutions for better returns to stakeholders.
INTRODUCTION
Jamun [Syzygium cumini (L.)] is a tropical fruit with an evergreen large tree belonging to Myrtaceae family. It is alternatively known as Eugenia jambolana in literature while jambolan, jamboo, doowet, Indian blackberry, faux pistachier, black plum, jamun, jambul and java plum are its common names. It is native to Himalayas, India, Australia, Malaysia, and Sri Lanka (Ayyanar and Subash-Babu, 2012). It is currently grown in China, India, Pakistan, Bangladesh, Sri Lanka, Israel, Eastern Australia, West Indies, Algeria, and USA (Oliveira et al., 2016). China is the largest producer followed by India and total production is about 13.5 m tonnes (Kishore, 2019). It has been adapted to wide environmental conditions and can tolerate water shortage and extensive flooding. Tropical climate with rocky and shallow soils are favourable for its cultivation (Sarvade et al., 2016). The most common way of propagation is through seeds and maiden bearing starts after 10 years of planation although maiden bearing could be possible after 5-6 years if propagated vegetatively (Singh et al., 2018).
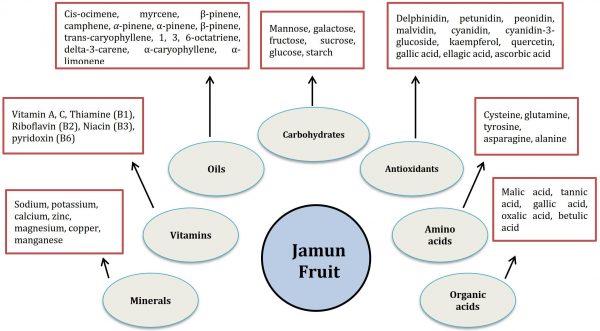
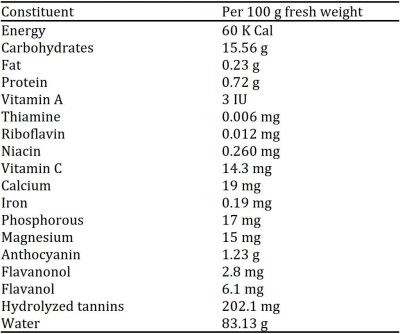
It is a rich source of phytonutrients including vitamins (thiamine, riboflavin, niacin, ascorbic acid), minerals (sodium, potassium, manganese, copper, calcium, iron, magnesium, zinc), free amino acids (cysteine, glutamine, tyrosine, asparagine, alanine), carbohydrates (mannose, galactose, fructose, sucrose, glucose) and proteins (Benherlal and Arumughan, 2007) (Fig. 1). Cis-ocimene, myrcene, β-pinene, camphene and α-pinene are essential oils obtained from jamun (Ayyanar and Subash-Babu, 2012). Different parts of Jamun tree have been used traditionally in folk medicines for curing several diseases including dysentery, diarrhoea, diabetes, and allergic issues due to their antibacterial, antiemetic, antipyretic, and anti-inflammatory properties (Dagadkhair et al., 2017). Fruit colour at immature stage is due to chlorophyll and carotenoids while there is higher anthocyanin biosynthesis with the fruit development (Singh et al., 2018). It contains higher number of antioxidants, phenolic contents and flavonoids making imperative for human consumption (Brito et al., 2007). Jamun shows astringency in its taste that may be either due to condensed tannins with higher molecular weight or these may be hydrolysed tannins with low molecular weight substances (Table 1).
Figure 1: The chemical composition / active ingredients showing the nutritional quality of jamun fruit (Baliga et al., 2011; Ayyanar and Subash-Babu, 2012; Singh et al., 2018).
Table 1: Nutritive value of jamun fruit.
(Baliga et al., 2011; Ayyanar and Subash-Babu, 2012; Singh et al., 2018)According to Patel and Rao (2014), jamun fruit exhibits significant rise in endogenous ethylene and respiration peaks representing behaviour like climacteric fruits. Fruit harvest and handling require a very careful practices as fruit is very delicate and perishable with 2-3 days shelf life at room temperature. Mechanical damage and physical bruising make fruit prone to the infestation of pests, pathogens, microbes, and sap flow provides host for quick fungal growth (Jain and Babbar, 2000). To extend its shelf life, the cold storage (8-10 °C) has widely been used which has improved its storability up to 3 weeks; however, some cultivars are susceptible to chilling injury when stored below 9 °C (Joshi, 2011). Many other alternatives like edible coatings, modified atmosphere packaging, heat treatment and chemical dipping treatments have been tested to increase its shelf life and maintain quality (Rai et al., 2011; Khaliq et al., 2020; Pattar et al., 2021b). Nevertheless, most of the work have been reported on value added products and juice instead of whole fruit storage. Postharvest diseases incidence is also limiting factor in its supply chain (Pattar et al., 2021a). Jamun fruit is being used by various processing industries and developing multiple products such as juice, drinks (ready to serve), squashes, wine, jam, cheese, toffee, and jelly etc (Dagadkhair et al., 2017).
To best of our knowledge, there is no published review article on production and postharvest technology of jamun fruit. Considering the gap, this article covers all aspects including preharvest factors affecting plant growth and fruit quality i.e., biochemical, and physiological changes during fruit ripening, harvest maturity indices, and postharvest physiology as well as processing technologies of jamun fruit.
PREHARVEST FACTORS AFFECTING GROWTH
Soil and climate
Jamun has variable adaptability in different climates and can be grown either in tropical or sub-tropical areas and can withstand water stress for a longer time (Chovatia and Singh, 2000). It is very hardy in nature and can bear very tough climatic conditions especially in semi-arid areas with poorly drained soils where other crops are unable to withstand and develop properly. Dry weather is required for optimum blooming and fruit set with subsequent rainfall that can increase fruit growth, improve fruit quality with better taste, maturity, colour, and size. Soil is the basic component for all types of vegetation and directly effects fruit yield and quality. Jamun needs well drained loamy soils for best growth; however, they can tolerate degraded lands, ravines, saline, and sodic soils (Bhowmik et al., 2013). Although the tree is very hardy but still there should not be very light or heavy soil for new plantation. Soil and water pH (up to 10.5) is also very critical factor for best emergence of new seedlings after plantation (Joshi, 2011).
Plant propagation and varieties
There are two types of plant propagations i.e., sexual through seeds and asexual propagation via grafting and micropropagation. Most common way of propagation in different areas of the world is through a seed that is source of genetic variability, helpful for maintaining good characters in changing environment. However, clonal conservation with required characteristics is not possible through this way of propagation and there is ample chance for crop improvement (Devi et al., 2002). The bearing period for sexually propagated plants starts from 8-10 years after propagation and this duration can be reduced by using asexual method of orchard establishment. Syzygium species has preferably used as rootstock for jamun production that can be raised in nurseries for further use. True-to-type tree with desirable characters is the main benefit of vegetative propagation; although, rootstock has very influential role in determining tree vigour, health, yield, and quality. In jamun, dwarfing rootstocks are more valuable because they provide manageable canopy that can ease cultural practices for the labour, also harvesting can be done with more efficiency than in taller plants. Insect attack especially termites’ resistance can be induced in jamun by using Syzygium densiflore as rootstock (Ayyanar and Subash-Babu, 2012). Rootstock nursery can be raised in polyethylene bags or soil for about 9-12 months to attain desirable plant height, health, and vigour. Sandy soil should be avoided as there is less than 10 % success rate. Grafting has higher success rate in jamun orchard development in contrast to other methods. Softwood should be used rather than hard wood for grafting, and veneer grafting is recommended in jamun on 9-12 months rootstock (Kishore, 2019). The other economical way to raise jamun is micropropagation that produces large scale plants in very short time and input. However, expertise is required for this method and there is chance of fungal infusion at propagated part of plant (Joshi, 2011). Jamun is referred as minor fruit without any varietal identification on genetic basis and every country has its own clones with traditional or local names. Typically, all varieties can be divided into two main types 1) kaatha or Desi jamun, 2) Ra or Ram jamun. Desi cultivars are blackish or dark purple in colour, slightly round and small sized fruit with acidic pulp, small stone and ripe in August, while Ram cultivars are sweet in taste with higher juice content and small size stone. Later one is more suitable for marketing in many areas of the world (Ghojage et al., 2011).
Irrigation and fertilizer management
Jamun is very hardy in nature that can tolerate severe drought as well as heavy rainfall and even flooding condition. However, young plants need regular irrigation after transplantation or emergence to cope with drought stress. As trees grow, there may be little or no need of irrigation for plant if there is adequate rainfall in the area of growth (Hiwale, 2015). Fertilizer requirements are directly associated with the tree age and soil fertility. The optimal production with good quality is only possible when there is 20-80 kg manure application for mature trees. The other important aspect is the time of fertilizer application and pre-flowering fertilizer dosage ensures full bearing with better fruit quality. Generally, there is no need of phosphorous for jamun with 400-500 g potassium and 600 g nitrogen per mature bearing tree; however, it may be different depending upon soils. The method of application is also key point for tree, and it is applied under the canopy followed by mixing in soil and irrigation. Loamy soils with good nutrient status negatively work against fertilizer application so it should be avoided with regular dose (Reang and Das, 2010).
Insect pests and diseases
Jamun is susceptible to insects and diseases that can lower its production and marketing. Generally, the major insects are white fly (Dialeurodes eugenia), leaf roller (Dudua aprobola Meyrick) and leaf eating caterpillars (Carea subtillis Walker) while major diseases are anthracnose, fruit rot and die back (Hiwale, 2015). White fly is most dangerous for jamun as they suck the fruit sap and bruise the skin leading to sap flow that is main point of contamination for disease attack. The attack can be more severe for plant facing drought stress leading to damage of twig or whole plant. Leaf roller incidence is second major issues during fruit growth and can be observed by rolled leaves with subsequent drying. This insect cause distortion of twigs and causes low leaf flush rate in affected trees. Leaf eating caterpillars feed on leave and causes defoliation of whole tree. The systematic collection can be used to control these insects or spray of recommended chemicals available in area can be used (Jain and Babbar, 2000). Fruit rot is major disease caused by Glomerella cingulata (Stonem) with primary symptoms on leaves exhibiting light brown to reddish colour scattered spots. Affected fruits develop water-soaked lesions which produce a scar on affected area with dark colour. Humidity or rain splashes enhance pathogen growth, and it can be controlled by using traditional Bordeaux mixture or recommended chemicals (Bose et al., 2001). Anthracnose caused by Colletotrichum species is another major disease in fruits that is developed mainly due to contaminated soils and spores can also be transferred by wind. Colletotrichum gloeosporioides is the major pathogen of jamun and fungicidal spray can control its growth (Hiwale, 2015).
FRUIT RIPENING PHYSIOLOGY
Fruit shows pure sigmoidal curve growth that can be divided into three distinct phases 1) slow growth stage start from fruit set to 7 weeks 2) rapid growth from 7-8 week after fruit set and 3) maturation phase with ethylene biosynthesis and respiration increase (Kishore, 2019). There is very slow fruit development in start with rapid increase in fruit weight and size in middle of development followed by maturation with no increase in size and colour development (Shukla an d Prasad, 1980). However, climatic conditions have direct role in fruit development time. There is series of biochemical changes during fruit development that involves the enzymatic activity that produces sweet, delicious, and soft fruits (Bajpai et al., 2012). Acidic contents of jamun are higher at green stage and there is characteristic decline with the advancement in growth as mature fruits have lower acidic value. Among organic acids, there is highest value of malic acid in ripe fruit with lower amount of oxalic acid (Sharma et al., 2012). Starch is the main sugar in immature green fruits that undergo hydrolysis with fruit development. On the other hand, there is escalation in non-reducing sugars that contribute sweet taste and fruit softening (Venkitakrishnan et al., 1997). Decline in acidity and increase in sugar content improves sugar to acid ratio leading to sweetness of fruit that is characteristic of ripe jamun. Fruit softening is brought about with the pectin degradation enzyme pectin methyl esterase which increases with the increase in fruit size with sudden decline at the time of maturation. Other enzyme is polygalacturonase that also reduces fruit hardness (Gol et al., 2015). Furthermore, ethylene biosynthesis and higher respiration rate also causes fruit softening. The sharp rise in ethylene with respiration rate at the time of maturation is the main reason to classify jamun as climacteric fruit; while other researchers reported it as non-climacteric fruit because there is no ripening in fruit after harvest from tree (Singh et al., 2018). Volatiles are responsible for fruit aroma so there have been about 30 compounds identified in ripe fruit; terpineol, myrcene, cis-ocimene and trans-cimene are major components. In addition, terpinyl valerate, geranyl butyrate and dihydrocarvyl acetate were detected as major esters responsible for aroma of jamun (Mehta et al., 2018).
Phenolic contents are important biochemical compounds present in jamun fruit mainly at immature stage with subsequent reduction at the time of maturity (Brandão et al., 2011). Kaempferol, quercetin, gallic acid and ellagic acid are the main phenolic content in jamun that have ability to scavenge the free radicals and contribute towards total antioxidants level. This scavenging and antioxidant level is much higher in peel tissues than fruit flesh and seeds (Bajpai et al., 2012). Carotenoids and chlorophyll contents are main ingredients in immature fruit due to which yellowish green colour is shown by fruit that undergo a decline with fruit development. Fruit colour of ripe jamun is due to lutein and total anthocyanin contents that produce dark purple colour at maturity (Veigas et al., 2007). Cyanide-3-glucoside, cyanidin, malvidin, peonidin, petunidine and delphinidin are major anthocyanins depending upon cultivars, climatic conditions, and analysis method (Benherlal and Arumughan, 2007). It is also dependent upon fruit development stage with minimum level at fruit set and highest at fully mature stage (Brito et al., 2007). There is direct correlation between ripening stage and anthocyanin accumulation rate and maximum increase is observable at fully mature stage (Lestario et al., 2017). Peonidin-3,5-O-diglucoside, malvidin-3,5-O-diglucoside, delphindin-3,5-O-diglucoside and cyanidin-3,5-O-diglucoside were exhibited by pink, green stage jamun although there was no anthocyanin detection at green stage. Moreover, peonidin-3,5-O-diglucoside and delphinidin-3-O-glucoside contents were present at light pink stage, and also major anthocyanin contents in jamun fruit. Tannins are other important components of immature and ripe fruits with two major types including proanthocyanidins or condensed tannins with large molecular size and galloylation degree account for astringency of fruit and second type is ellagitannins or hydrolysable tannins (Pattar et al., 2021a)[/fusion_one_page_text_link. Later one is very complex molecule having complex constituents like valoneic acid or trisalloyl, nonahydroxytriphenolyl (NHTP), hexahydroxydiphenoyl (HHDP) and gallic acid. Tong et al. (2014) outlined that valoneic acid dilactone methyl ester, ellagic acid and gallic acid are major hydrolysable tannins, are the part of fruit pulp and peel. They show characteristic decline in ripe fruit as maximum are observed at immature green stage (Tavares et al., 2016).
FRUIT MATURITY AND HARVEST HANDLING
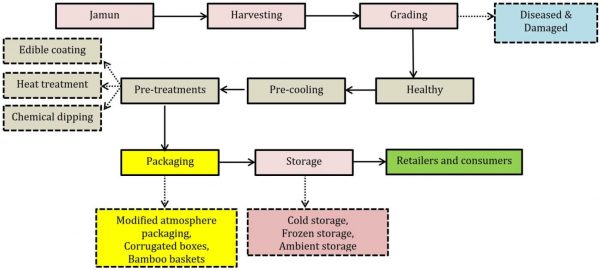
Fruit maturity can be assessed on different indicators like fruit colour, acidity, sugar contents, anthocyanin, and phenolic contents. However, the most common method is the fruit colour on the basis of which harvesting is done. There is discontinuous ripening of fruits in same inflorescence so there may be difference in maturity with multiple pickings. Fully dark purple colour stage is the indicator of harvesting stage (Venkitakrishnan et al., 1997). Ethylene biosynthesis and respiration increase can also be used as the maturity index. Fruit softening is another very important harvest maturity index that is actually due to pectin methyl esterase and polygalacturonase enzymes activities subjected to degradation of pectin content in cell wall (Gol et al., 2015). In India, fruit taste is also considered as the quality and maturity indicator, harvesting is done when fruit becomes sweet and non-astringent due to reduction in tannin contents (Patel and Rao, 2014). The most common method is shaking of branch and fruits drop on cloth underneath tree. Other way is hand picking that is very laborious work due to large tree size and delicate branches that can be broken down during labour climbing. Jamun fruit is very perishable and harvesting by shaking often causes fruit mechanical damage that is point of contamination for microbes on the sap flow. Packing is mostly done in the field however pre-cooling at 8 °C can enhance fruit postharvest shelf life (Shukla and Prasad, 1980). Fruits are sorted manually on the basis of fruit size, weight, colour, mechanical damage, and diseases. Harvesting is usually done on daily basis with immediate transport in local markets with corrugated boxes or bamboo baskets (Joshi, 2011). The proposed operations from harvest to marketing have been described in Fig. 2.
Figure 2: Flow chart for postharvest handling and processing techniques of jamun fruit as per available published literature.
POSTHARVEST TECHNOLOGY OF JAMUN FRUIT
Cold storage
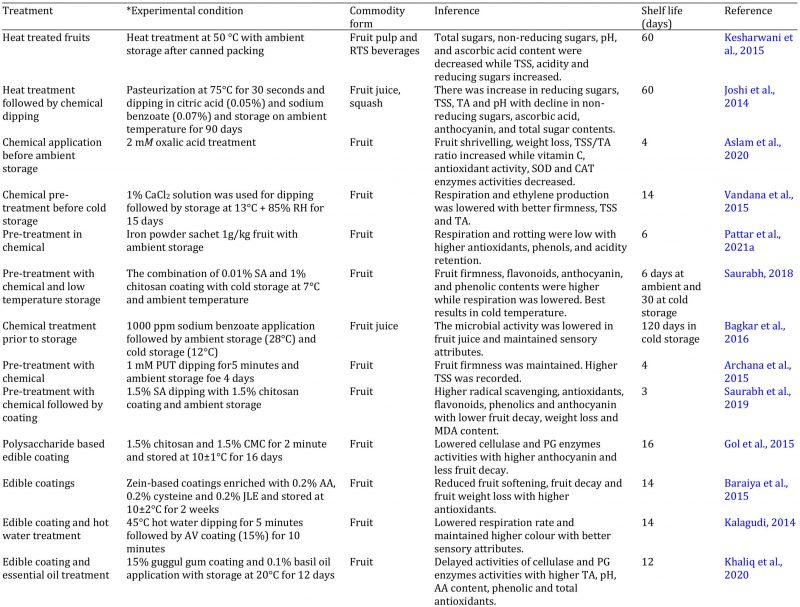
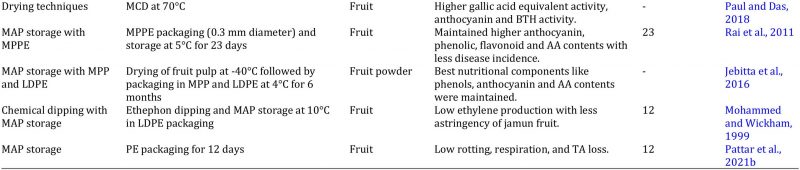
Jamun fruit is very perishable, and its shelf life is about 3 days on ambient temperature because it ripe only on tree and there is no change in fruit colour after harvesting; while it takes about 12 hours from harvest to local markets (Ali et al., 2015). Many techniques have been used to maintain its postharvest quality (Table 2). Cold storage is one of the techniques to enhance the shelf life of jamun fruit above 10 °C because fruit softening is carried out when stored below this temperature (Tong et al., 2014). Cold storage lowers respiration and ethylene production. Quality attributes and nutritional value is also retained during cold storage with less softening of fruit for 5 days (Shahnawaz et al., 2009). Similarly, sugar contents and acidity can be increased during cold storage with better sensory attributes (Priyanka et al., 2015). The use of jamun fruit is very limited and its juice is also prepared for marketing due to high nutritive value. The main issue in jamun fruit juice is quality deterioration and unpleasant smell due to microbial infestation. Cold storage with sodium benzoate application-maintained fruit juice quality in optimum range with less microbial infestation for 4 months (Bagkar et al., 2016). Moreover, heat treatment of juice followed by cold storage can enhance its shelf life for 60 days with decrease in anthocyanin content, total sugars and non-reducing sugars while reducing sugars, pH and TSS are increased. However, Kesharwani et al. (2015) observed decline in pH and ascorbic acid in jamun fruit juice when stored more than 15 days during cold storage.
Table 2: Effect of different treatments on the quality management of jamun fruit and products.
AA = ascorbic acid, AV = aloe vera, BTH = butylated hydroxyanisole, CAT = catalase, CMC = carboxymethyl cellulose, HDPE = high density polyethylene, LDPE = low density polyethylene, MAP = modified atmosphere packaging, MCD = microwave convective hot air drying, MDA = malondialdehyde, mM = milli mole, MPP = metalized polyester pouch, MPPE = macro perforated polyethylene, PG = polygalacturonase, ppm = parts per million, PUT = putrescine, RH = relative humidity, RTS = ready to serve, SA = salicylic acid, SOD = superoxide dismutase, TA = titratable acidity, TSS = total soluble solids.
Anti-ripening chemicals
Chemical application is one of the most widely used methods to preserve the fruits and shelf-life extension. They not only maintained physico-chemical attributes but also enhance their shelf life and storage quality (Shah et al., 2017). Calcium chloride (CaCl2) is very important chemical used for postharvest quality management of horticultural commodities. It can increase the shelf life of jamun fruit up to 14 days with best organoleptic attributes and decrease in microbial decay (Vandana et al., 2015). The combination of CaCl2 with GA3 significantly enhanced total antioxidant level and phenolic contents in jamun. The fruit rotting and fruit firmness decline was also controlled by this treatment during 6 days storage at ambient temperature (Pattar et al., 2021a). The combination of salicylic acid with chitosan followed by low temperature storage maintained higher antioxidant level, radical scavenging activity, flavonoids, and ascorbic acid contents with reduction in weight loss, anthocyanin degradation and firmness loss for 6 days at ambient; while similar results were achieved at 7 °C for 30 days without causing any chilling injury in fruits (Saurabh, 2018). Similar treatment in another study was effective in lowering microbial decay and anthocyanin degradation (Saurabh et al., 2019). Application of oxalic acid dip treatment was also effective to control fruit decay, anthocyanin degradation and fruit weight loss with higher phenolic and ascorbic acid contents (Aslam et al., 2020). Metabolic energy conservation is key factor in fruit quality conservation and higher energy status of fruit can effectively delay the senescence. Higher respiration rate catalyses fruit senescence and shelf life is ultimately reduced due to higher metabolic activity. Archana et al. (2015) reported that application of putrescine lowered respiration rate, fruit firmness loss and fruit weight loss after 4 days storage.
Edible coatings
The edible coating works on the principle of modifications in the internal metabolism of the commodity when applied externally. The thin layer of edible coating develops a modified atmosphere inside the subjected fresh produce which restricts gaseous exchange thereby delaying the postharvest ripening and senescence during the storage period (Hasan et al., 2021). Due to chemical residual effects, there was increase in use of edible coatings that have no harmful effects on consumer health due to their organic nature (Xing et al., 2019). It provides protection against pathogens and minimizes the fruit quality losses by improving physic-chemical attributes. Chitosan application was helpful in lowering fruit damage in jamun for 30 days during cold storage and there was increase in non-reducing sugars as storage progressed (Saurabh, 2018). Meanwhile, there was less malondialdehyde (MDA) production due to suppression of lipid peroxidation by chitosan and salicylic acid (Saurabh et al., 2019). The combination of chitosan with carboxymethyl cellulose and alginate reduced oxidative stress by maintaining higher radical scavenging activity for 16 days at ambient temperature (Gol et al., 2015). These beneficial effects can be increased by applying exogenous antioxidants along with edible coating. The exogenous application along with internal antioxidant biosynthesis can alleviate the senescence of fruit with better biochemical profile. Baraiya et al. (2015) reported higher antioxidant level with alleviated fruit softening when zein based coating was incorporated with jamun leave extract, ascorbic acid, and cysteine. However, decay was observed after 6 days in both treated and untreated fruits. Aloe vera gel is most reported plant-based coating for shelf life and quality management of fruits. It improves fruit quality and shelf life with no harmful effects on consumer health. The fruit colour (L*, a*, b*, C*, h°) and TSS was maintained in aloe vera gel coated, neem leaf extract and CaCl2 treated fruits with extended shelf life for up to 15 days without any fruit decay incidence (Kalagudi, 2014). Guggal gum coating in jamun maintained fruit firmness by lowering polygalacturonase and cellulose enzymes activities for 12 days ambient temperature storage; however, there was no difference in fruit sensory attributes in untreated and treated fruits (Khaliq et al., 2020).
Heat treatments
Heat treatments either by hot air or hot water treatment have been investigated for increase in shelf and postharvest quality of horticultural commodities (Jin et al., 2016). Jamun is very perishable fruit, and its shelf life is about 3 days after harvest so different products have been formed by drying either through vacuum drying, hot air or freeze drying. The level of phenolic contents and ascorbic acid was more in dried than fresh fruit samples. Moreover, hot air drying is most successful method to maintain dried jamun fruit products than vacuum or freeze drying (Paul and Das, 2018). The colour variation (ΔE*) in hot air dried jamun is also less than sun drying (Paul and Das, 2019). However, the anthocyanin content is reduced due to hot air application and ultrasonic pasteurization was found better than heat treatment. There was about 21.9% anthocyanin degradation in ultrasonic pasteurized jamun while this reduction was about 34.5% in hot air treated fruits. The other disadvantage of heat application on jamun is loss of enzymatic activity that may deteriorate fruit quality when stored as dried (Shaheer et al., 2014).
Modified atmosphere packaging (MAP)
Marketing of jamun is usually done in corrugated boxes and bamboo stick baskets. Proper packaging can increase fruit shelf life, maintain quality, avoid mechanical bruising, and increase marketing time (Pattar et al., 2021b). Modified atmosphere packaging is very important marketing technique that enhance fruit marketing window with minimum losses of jamun. Rai et al. (2011) evaluated the effectiveness of MAP by using non-perforated and perforated polyethylene packaging for jamun shelf life. They outlined that storage capacity can be increased for 23 days by using macro-perforated polyethylene packaging for jamun fruit subjected to 70-80 % relative humidity and 5 °C storage temperature. The microbial infestation was lowered by using polyethylene with 1 µm perforation. The comparison analysis indicated that better phenolic, anthocyanin and ascorbic acid contents were retained by using low density packaging than high density polyethylene (Jebitta et al., 2016). The astringency of jamun fruit was reduced by using ethephon treatment followed by low density polyethylene packing and improved consumer demand and acceptability (Mohammed and Wickham, 1999). Fruit rotting, ethylene biosynthesis and respiration rate was delayed by polyethylene perforated packaging (Pattar et al., 2021b).
CONCLUSION AND FUTURE PROSPECTS
Jamun is prime fruit growing around the globe, though considered as minor and underutilized crop due to several constraints such as lower production, limited shelf life, no postharvest technology protocol, and unsustainable marketing system during supply chain. It has low shelf life of about 3 days but pre-treatments like edible coating, heat treatments and chemical dipping in combination with polyethylene packaging can increase its marketing window. However, there is limited material available on enzymatic activities to modulate ethylene production and respiration rate. It has been noted that the jamun crop has great potential to be studied for various aspects including molecular characterization of gene pool, the release of commercial varieties for cultivation with better yield, genetic modulation for reducing pit size with increased flesh proportion, preharvest factors affecting quality, postharvest quality preservation and processing to develop new value-added products. Moreover, additional research is required to improve processing techniques and standardization of pre-treatments on jamun fruit. The cultivar identification using genetic markers may also be done to domesticate the wild cultivars.
Acknowledgements
The authors dedicated this review article to world-renowned postharvest professional Late Prof. Adel A. Kader for his phenomenal contribution to the horticulture industry around the globe.
Declaration of competing interests
The authors claim no conflict of interest whether financial or another aspect.
REFERENCES
Ali, S. T. M., Abbasi, K. S., Ali, A. and Hussain, A. 2015. Some compositional and biochemical attributes of jaman fruit (Syzygium cumini L.) from Potowar region of Pakistan. Research in Pharmacy, 3(5): 1-9. [Abstract/FREE full text, Google Scholar, CrossRef]
Archana, T. J., Suresha, G. J., Vandana, A. K. and Swamy, G. S. K. 2015. Effect of exogenous application of putrescine on storage behaviour of jamun (Syzygium cumini Skeels) fruits. Acta Horticulturae, 1241: 577-582. [Abstract/FREE full text, Google Scholar, CrossRef]
Aslam, M. M., Ullah, S., Razzaq, K., Rajwana, I. A., Akhtar, G., Nazar, H., Faried, M. A., Ullah, U. N. and Khalil, U. 2020. Orchard locality and postharvest oxalic acid application influence fruit quality and shelf life of Jamun (Syzygium cumini L.) fruit. Journal of Pure and Applied Agriculture, 5(4): 34-41. [Abstract/FREE full text, Google Scholar, CrossRef]
Ayyanar, M. and Subash-Babu, P. 2012. Syzygium cumini (L.) Skeels: A review of its phytochemical constituents and traditional uses. Asian Pacific Journal of Tropical Biomedicine, 2(3): 240-246. [Abstract/FREE full text, Google Scholar, CrossRef]
Bagkar, P. P., Khandekar, R. G., Kadam, J. J., Pawar, C. D. and Patil, R. S. 2016. Effect of sodium benzoate concentrations and storage conditions on sensory evaluation and microbial count of Jamun juice during storage. Journal of the Indian Society of Coastal Agricultural Research, 34(2): 79-84. [Abstract/FREE full text, CrossRef]
Bajpai, A., Singh, A. K. and Ravishankar, H. 2012. Reproductive phenology, flower biology and pollination in Jamun (Syzygium cuminii L.). Indian Journal of Horticulture, 69(3): 416-419. [Abstract/FREE full text, Google Scholar, CrossRef]
Baliga, M.S., Bhat, H.P., Baliga, B.R.V., Wilson, R. and Palatty, P.L. 2011. Phytochemistry, traditional uses and pharmacology of Eugenia jambolana Lam. (black plum): A review. Food Research International, 44(7): 1776-1789. [Abstract/FREE full text, Google Scholar, CrossRef]
Baraiya, N. S., Rao, T. V. R. and Thakkar, V. R. 2015. Improvement of postharvest quality and storability of jamun fruit (Syzygium cumini L. Var. Paras) by zein coating enriched with antioxidants. Food and Bioprocess Technology, 8(11): 2225-2234. [Abstract/FREE full text, Google Scholar, CrossRef]
Benherlal, P. S. and Arumughan, C. 2007. Chemical composition and in vitro antioxidant studies on Syzygium cumini fruit. Journal of the Science of Food and Agriculture, 87(14): 2560-2569. [Abstract/FREE full text, Google Scholar, CrossRef]
Bhowmik, D., Gopinath, H., Kumar, B. P. and Kumar, K. 2013. Traditional and medicinal uses of Indian black berry. Journal of Pharmacognosy and Phytochemistry, 1(5). [Abstract/FREE full text, Google Scholar, CrossRef]
Bose, T. K., Mitra, S. K. and Sanyal, D. 2001. Fruits: tropical and subtropical. Naya Parkashan, Calcutte. [Abstract/FREE full text, Google Scholar, CrossRef]
Brandão, T. S. D. O., Sena, A. R. D., Teshima, E., David, J. M. and Assis, S. A. 2011. Changes in enzymes, phenolic compounds, tannins, and vitamin C in various stages of jambolan (Syzygium cumini Lamark) development. Food Science and Technology, 31: 849-855. [Abstract/FREE full text, Google Scholar, CrossRef]
Brito, E. S., De Araújo, M. C. P., Alves, R. E., Carkeet, C., Clevidence, B. A. and Novotny, J. A. 2007. Anthocyanins present in selected tropical fruits: acerola, jambolão, jussara, and guajiru. Journal of Agricultural and FOOD chemistry, 55(23): 9389-9394. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Chovatia, R. S. and Singh, S. P. 2000. Effect of time on budding and grafting success in jamun (Syzygium cumini Skeel). Indian Journal of Horticulture, 57(3): 255-258. [Abstract/FREE full text, Google Scholar]
Dagadkhair, A. C., Pakhare, K. N., Todmal, A. D. and Andhale, R. R. 2017. Jamun (Syzygium cumini) Skeels: a traditional therapeutic tree and its processed food products. International Journal of Pure and Applied Bioscience, 5(5), 1202-1209. [Google Scholar, CrossRef]
Devi, S. P., Thangam, M., Desai, A. R. and Adsule, P. G. 2002. Studies on variability in physico-chemical characters of different jamun (Syzygium cumini) accessions from Goa. Indian Journal of Horticulture, 59(2): 153-156. [Abstract/FREE full text, Google Scholar]
Ghojage, A. H., Swamy, G. S. K., Kanamadi, V. C., Jagdeesh, R. C., Kumar, P., Patil, C. P. and Reddy, B. S. 2011. Studies on variability among best selected genotypes of jamun (Syzygium cumini Skeels.). Acta Horticulturae, 890: 255-260. [Abstract/FREE full text, Google Scholar, CrossRef]
Gol, N. B., Vyas, P. B. and Ramana Rao, T. V. 2015. Evaluation of polysaccharide-based edible coatings for their ability to preserve the postharvest quality of Indian blackberry (Syzygium cumini L.). International Journal of Fruit Science, 15(2): 198-222. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Hasan, M. U., Riaz, R., Malik, A. U., Khan, A. S., Anwar, R., Rehman, R. N. U. and Ali, S. 2021. Potential of Aloe vera gel coating for storage life extension and quality conservation of fruits and vegetables: An overview. Journal of Food Biochemistry, 45(4), e13640. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Hiwale, S. 2015. Sustainable horticulture in semiarid dry lands. Springer India. pp. 135-152. [Abstract/FREE full text, Google Scholar, CrossRef]
Jain, N. and Babbar, S. B. 2000. Recurrent production of plants of black plum, Syzygium cuminii (L.) Skeels, a myrtaceous fruit tree, from in vitro cultured seedling explants. Plant Cell Reports, 19(5): 519-524. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Jebitta, S. R., Ramanathan, M., Rajkumar, P. and Jebitta, S. R. 2016. Physical and nutritional evaluation of freeze dried Jamun pulp powder stored at different package and storage conditions. Indian Journal of Natural Sciences, 7: 39. [Abstract/FREE full text, Google Scholar, CrossRef]
Jin, P., Zheng, C., Huang, Y. P., Wang, X. L., Luo, Z. S. and Zheng, Y. H. 2016. Hot air treatment activates defense responses and induces resistance against Botrytis cinerea in strawberry fruit. Journal of Integrative Agriculture, 15(11): 2658-2665. [Abstract/FREE full text, Google Scholar, CrossRef]
Joshi, H. N., Hiwale, S. S. and Leua, H. N. 2014. Studies on physico-chemical characteristics of jamun (Syzygium cuminii Skeels) beverages during storage. Madras Agricultural Journal, 101. [Abstract/FREE full text, Google Scholar]
Joshi, P. V. 2011. Post harvest handling and marketing of Jamun (Syzygium cuminii) in Sindhdurg District of Maharashtra state. International Journal of Commerce and Business Management, 4(2): 249-253. [Abstract/FREE full text, Google Scholar, CrossRef]
Kalagudi, V. A. 2014. Influence of postharvest treatments on quality and shelf life of jamun (Syzygium cumini Skeels.) fruits under cold storage (Doctoral dissertation, KRC College of Horticulture, Arabhavi. University of Horticultural Sciences, Bagalkot. [CrossRef]
Kesharwani, A., Dikshit, S. N., Kumar, K., Thakur, P. and Chandel, N. 2015. Studies on physico-chemical composition of jamun and changes in chemical composition of RTS beverage during storage. The Bioscan, 7:379-383. [Abstract/FREE full text, Google Scholar, CrossRef]
Khaliq, G., Saleh, A., Bugti, G. A. and Hakeem, K. R. 2020. Guggul gum incorporated with basil essential oil improves quality and modulates cell wall-degrading enzymes of jamun fruit during storage. Scientia Horticulturae, 273: 109608. [Abstract/FREE full text, Google Scholar, CrossRef]
Kishore, K. 2019. Phenological growth stages and heat unit requirement of Indian blackberry (Syzygium cumini L., Skeels). Scientia Horticulturae, 249:455-460. [Abstract/FREE full text, Google Scholar, CrossRef]
Lestario, L. N., Howard, L. R., Brownmiller, C., Stebbins, N. B., Liyanage, R. and Lay, J. O. 2017. Changes in polyphenolics during maturation of Java plum (Syzygium cumini Lam.). Food Research International, 100: 385-391. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Mehta, P. K., de Sousa Galvão, M., Soares, A. C., Nogueira, J. P. and Narain, N. 2018. Volatile constituents of jambolan (Syzygium cumini L.) fruits at three maturation stages and optimization of HS-SPME GC-MS method using a central composite design. Food Analytical Methods, 11(3): 733-749. [Abstract/FREE full text, Google Scholar, CrossRef]
Mohammed, M. and Wickham, L. 1999. Effect of modified atmosphere packaging and ethanol on the deastringency process in jamun (Syzygium cuminii) fruit. Journal of Applied Horticulture, 1(2): 105-107. [Abstract/FREE full text, Google Scholar, CrossRef]
Oliveira, É. R., Caliari, M., Soares Júnior, M. S. and Vilas Boas, E. V. D. B. 2016. Bioactive composition and sensory evaluation of blended jambolan (Syzygium cumini) and sugarcane alcoholic fermented beverages. Journal of the Institute of Brewing, 122(4): 719-728. [Abstract/FREE full text, Google Scholar, CrossRef]
Patel, P. R. and Rao, T. R. 2014. Growth and ripening in black plum [Syzygium cumini (L.) Skeels]. International Journal of Fruit Science, 14(2):147-156. [Abstract/FREE full text, Google Scholar, CrossRef]
Pattar, A., Laxman Kukanoor, D. K., Naik, N., Jholgikar, P., Koulagi, S. and Naika, M. B. 2021a. Influence of post-harvest treatments on quality and shelf life of jamun (Syzygium cuminii Skeels.) fruits under ambient storage. The Pharma Innovation Journal, 10(1), 523-528. [Abstract/FREE full text, CrossRef]
Pattar, A., Laxman Kukanoor, D. K., Naik, N., Jholgikar, P., Koulagi, S. and Naika, M. B. 2021b. Effect of different packaging materials and storage condition on quality of jamun (Syzygium cuminii Skeels) fruits. Journal of Pharmacognosy and Phytochemistry, 10(1):928-932. [CrossRef]
Paul, I. D. and Das, M. 2018. Effect of freeze, microwave-convective hot air, vacuum and dehumidified air drying on total phenolics content, anthocyanin content and antioxidant activity of jamun (Syzygium cumini L.) pulp. Journal of Food Science and Technology, 55(7): 2410-2419. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Paul, I. D. and Das, M. 2019. Microwave‐convective hot airdried jamun (Syzygium cumini L.) pulp powder: Optimization of drying aids. Journal of Food Process Engineering, 42(6): e13166. [Abstract/FREE full text, Google Scholar, CrossRef]
Priyanka, N., Dorajeerao, A. V. D., Sudhavani, V. and Umakrishna, K. 2015. Physico-chemical characters and sensory evaluation of jamun based blended squash beverages during storage. Plant Archives, 15(2): 939-946. [Google Scholar, CrossRef]
Rai, D. R., Chadha, S., Kaur, M. P., Jaiswal, P. and Patil, R. T. 2011. Biochemical, microbiological and physiological changes in Jamun (Syzyium cumini L.) kept for long term storage under modified atmosphere packaging. Journal of Food Science and Technology, 48(3): 357-365. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Reang, E. and Das, B. C. 2010. Effect of different manures and fertilizers combination on growth behavior of jamun (Syzygium cuminii Skeels) cv local. Environment and Ecology, 28(4): 2285-2290. [Abstract/FREE full text, Google Scholar, CrossRef]
Sarvade, S., Gautam, D. S., Bhalawe, S. and Bisen, P. K. 2016. An overview of potential multipurpose agroforestry tree species, Syzygium cuminii (L.) Skeels in India. Journal of Applied and Natural Science, 8(3): 1714-1719. [Abstract/FREE full text, Google Scholar, CrossRef]
Saurabh, V. 2018. Modulation of postharvest quality and senescence of jamun (Syzygium cumini Skeels) fruit by salicylic acid and chitosan treatment (Doctoral dissertation, Department of Horticulture, Institute of Agricultural Sciences, Banaras Hindu University Varanasi). [CrossRef]
Saurabh, V., Barman, K. and Singh, A. K. 2019. Synergistic effect of salicylic acid and chitosan on postharvest life and quality attributes of jamun (Syzygium cumini Skeels) fruit. Acta Physiologiae Plantarum, 41(6): 1-11. [Abstract/FREE full text, Google Scholar, CrossRef]
Shah, H. M. S., Khan, A. S. and Ali, S. 2017. Pre-storage kojic acid application delays pericarp browning and maintains antioxidant activities of litchi fruit. Postharvest Biology and Technology, 132: 154-161. [Abstract/FREE full text, Google Scholar, CrossRef]
Shaheer, C. A., Hafeeda, P., Kumar, R., Kathiravan, T., Kumar, D. and Nadanasabapathi, S. 2014. Effect of thermal and thermosonication on anthocyanin stability in jamun (Eugenia jambolana) fruit juice. International Food Research Journal, 21(6): 2189. [Abstract/FREE full text, Google Scholar, CrossRef]
Shahnawaz, M., Sheikh, S. A. and Nizamani, S. M. 2009. Determination of nutritive values of Jamun fruit (Eugenia jambolana) products. Pakistan Journal of Nutrition, 8(8): 1275-1280. [Abstract/FREE full text, Google Scholar, CrossRef]
Sharma, S., Mehta, B. K., Mehta, D., Nagar, H. and Mishra, A. 2012. A review on pharmacological activity of Syzygium cumini extracts using different solvent and their effective doses. International Research Journal of Pharmacy, 54(3): 12. [Abstract/FREE full text, Google Scholar]
Shukla, J. P. and Prasad, A. 1980. Changing pattern of jamun fruit during growth and development III. Changes in respiratory activity. Progressive Horticulture, 12: 71-73. [Abstract/FREE full text]
Singh, B., Singh, J. P., Kaur, A. and Singh, N. 2018. Insights into the phenolic compounds present in jambolan (Syzygium cumini) along with their health‐promoting effects. International Journal of Food Science & Technology, 53(11): 2431-2447. [Abstract/FREE full text, Google Scholar, CrossRef]
Tavares, C. I. M., Lago-Vanzela, E. S., Rebello, L. P. G., Ramos, A. M., Gomez-Alonso, S., Garcia-Romero, E., Da-Silva, R. and Hermosin-Gutierrez, I. 2016. Comprehensive study of the phenolic composition of the edible parts of jambolan fruit (Syzygium cumini (L.) Skeels). Food Research International, 82:1-13. [Abstract/FREE full text, Google Scholar, CrossRef]
Tong, W. Y., Wang, H., Waisundara, V. Y. and Huang, D. 2014. Inhibiting enzymatic starch digestion by hydrolyzable tannins isolated from Eugenia jambolana. LWT-Food Science and Technology, 59(1): 389-395. [Abstract/FREE full text, Google Scholar, CrossRef]
Vandana, A. K., Suresha, G. J. and Swamy, G. S. K. 2015. Impact of calcium chloride pre-storage treatment on jamun (Syzygium cumini Skeels) fruits under cold storage. The Bioscan, 10(1): 199-202. [Google Scholar, CrossRef]
Veigas, J. M., Narayan, M. S., Laxman, P. M. and Neelwarne, B. 2007. Chemical nature, stability and bioefficacies of anthocyanins from fruit peel of Syzygium cumini Skeels. Food Chemistry, 105(2): 619-627. [Abstract/FREE full text, Google Scholar, CrossRef]
Venkitakrishnan, M., Panjatcharam, V., Kumaravelu, G. and Ramanujam, M. P. 1997. Physico-chemical changes during maturation and ripening of jambolan fruit. Indian Journal of Plant Physiology, 2: 267-270. [Google Scholar, CrossRef]
Xing, Y., Li, W., Wang, Q., Li, X., Xu, Q., Guo, X., Bi, X., Liu, X., Shui, Y., Lin, H. and Yang, H. 2019. Antimicrobial nanoparticles incorporated in edible coatings and films for the preservation of fruits and vegetables. Molecules, 24(9): 1695. [Abstract/FREE full text, Google Scholar, CrossRef]
Antioxidants, anti-ripening, biochemical changes, perishable, Jamun industry.
* Corresponding author
a Education Office Samundri, School Education Department, Government of Punjab (37300), Pakistan
b SVVCP, Centre for Agriculture and Bioscience International, Pakistan
Email: shoaib_agrarian@hotmail.com (H.M.S. Shah)
This article does not contain any abbreviations to display here.
Received: 30 February 2021
Revised: 13 June 2021
Accepted: 08 July 2021
Published: 30 September 2021
How to Cite
| AMA |
Shah HMS, Hasan MU, Waheed A. Jamun (Syzygium cumini): An underutilized potential fruit crop in Asia. J Hortic Sci Technol. 2021;4(3):109-117. doi:https://doi.org/10.46653/jhst2143109
|
| MLA |
Shah, Hafiz Muhammad Shoaib, et al. “Jamun (Syzygium Cumini): An Underutilized Potential Fruit Crop in Asia.” Journal of Horticultural Science & Technology, vol. 4, no. 3, 1, 2021, pp. 109–17, https://doi.org/10.46653/jhst2143109.
|
| APA |
Shah, H. M. S., Hasan, M. U., & Waheed, A. (2021). Jamun (Syzygium cumini): An underutilized potential fruit crop in Asia. Journal of Horticultural Science & Technology, 4(3), 109–117. https://doi.org/10.46653/jhst2143109
|
Download Citation (RIGHT CLICK & “SAVE LINK AS”)
This article do not contain any supplementary data.