Copyright: © 2021 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution 4.0 International License. J. Hortic. Sci. Technol. © 2021 Pakistan Society for Horticultural Science.
ABSTRACT
The cut flower industry is continuously progressing due to their increasing demand at national and international levels. New flowers like sunflower (Helianthus annuus L) and many others are added to this category to make this industry more profitable. Due to nutritional and aesthetic value, sunflower has attained great consideration for floriculture dietary needs. However, their vase life is short. The vase life of sunflowers varies between 4-13 days, depending on the cultivar. Vase life problems refer to the challenges and limitations associated with maintaining the freshness and longevity of cut flowers in a vase or floral arrangement. The current study was carried out to enhance the vase life of cut sunflowers by using various vase life-improving solutions. In this study, we investigated the effect of different vase solutions on cut sunflowers. The flowers were treated with six different solutions: silver nitrate (17%), Disprin (2 mM Aspirin), rosewater (8%), Sprite (50%), chitosan (2.5%), and distilled water as a controlled treatment. The vase life of the sunflowers was measured, and the results showed that the flowers treated with the Sprite (50%) had taken the highest number of days for petal dropping (7.4 days), flower wilting (9 days), petal wilting (6.8 days). The highest relative water uptake (162.8 mL) by flowers was also observed in the Sprite. The highest number of days taken for petal discolouration (7.8 days), stem bending (8 days) and leaf wilting (8.8 days) was observed in distilled water. Whereas, the highest number of days for stem discolouration (8 days) was observed in rosewater. In conclusion, Sprite shows the best results, and then distilled water compared to other treatments.
INTRODUCTION
Cut flowers are valuable horticultural commodities. Production of high-quality cut flowers on a sustainable basis and prolonging their vase life are crucial and pragmatic for producing items that meet market standards. Most of the studies were conducted on the vase-life of cut flowers (da Costa et al., 2021). In the floral industry, cut sunflowers (Helianthus annuus L.) have grown in economic significance (Sharif et al., 2019). Their vase life is less. Depending on the cultivar, sunflowers may have a vase life of 4–13 days. After harvest, scape bending, abscission, and withering of the ray flowers occur (Faust et al., 2021). According to Avilala et al. (2021), merchants in both domestic and foreign markets favour cut flowers with a longer vase life since it is regarded as a crucial quality attribute in flower production. The strong, ridged architecture and many coarse hairs on sunflower stems make it simple for germs to adhere to the stem and then spread to the vase solution. Therefore, because bacteria obstruct the xylem in the stem, cut sunflowers might be considered vulnerable to water stress (Kılıç et al., 2020).
The lifespan of a flower vase has an impact on the economy. Short vase life can be caused by several things, including ethylene production during the climacteric stage (Ha et al., 2019) and/or bacterial and degradation product-induced occlusion of stem xylem arteries (Li et al., 2017). Because they compress DNA molecules, weaken the cytoplasmic membrane, and loosen cell walls, silver ions (Ag+) efficiently destroy microorganisms (Carrillo-Lopez et al., 2016). Synthetic germicides like silver acetate (AgC2H3O2) or silver nitrate (AgNO3) are used in commercially available floral preservatives to limit microbial development or aqueous microflora (Rahman et al. 2018). One of the most often utilized silver salts in floral preservatives is silver nitrate, employed as an ethylene binding inhibitor and anti-microbial in commercial products (Manzoor et al., 2021).
Given that chitosan and its derivatives have fungicidal properties and induce defense mechanisms in plant tissues, it has been suggested that using them can extend the postharvest life of several horticultural commodities (Katiyar et al., 2015). A biopolymer called chitosan is created when chitin is deacetylated (Philibert et al., 2017). According to Hong-juan and Huan-qing (2015), covering cut roses with chitooligosaccharides, a chitosan derivative, increased vase life by 6.4 days compared to the control treatment. Reducing the pH in preservative formulations is a major function of organic acids. Citric acid is typically used to reduce the pH of gladiolus (G. grandiflorus L.) vase solutions (Uddin et al., 2016). It was discovered that carnations responded well to a pre-shipment treatment in which 150 ppm of citric acid was added to the pulse solution. By adjusting the pH of the cell sap, citric acid boosts the intensity of petal colour and avoids vascular bundle blockage. It also improves water balance. Citric acid, used in the holding solution at a concentration of 0.5–0.7%, encouraged the growth of flowers and preserved the cut tuberose spikes’ quality (Akram et al., 2021).
The physiological functions of plants are regulated by salicylic acid (SA), an endogenous growth regulator of phenolic origin. According to experimental findings, SA participates in signal modulation of gene expression during Arabidopsis leaf senescence (Wang et al., 2021). Additionally, SA may decrease fruit ripening (Sun et al., 2021), regulate gravitropism (Ke et al., 2021), and modulate other processes. In addition to inducing systemic acquired resistance (SAR) in plants, SA is a well-known naturally occurring signalling molecule that is essential for initiating and signalling a defense response against a variety of pathogenic diseases (Saleem et al., 2021). After a localized infection, some sort of mediator for long-distance communication is needed to induce SAR. According to a review of the literature, salicylic acid travels through the phloem from plant parts that are diseased to those that are not (David et al., 2019). Using radiolabelled SA or its analogues, these results were further validated (Azhir et al., 2015).
This study aimed to evaluate the impact of different holding solutions on the vase life of cut sunflowers.
MATERIALS AND METHODS
Mature sunflowers were harvested from the Cut Flower and Vegetable Research and Training Cell at The Islamia University of Bahawalpur. The ornamental sunflower sticks were approximately 50-55 cm in height, each containing an average of three leaves. After the harvest, the sticks were immediately placed in a bucket of tap water and transported to the laboratory of the Department of Horticultural Sciences. At the laboratory, all flower sticks received a slant recut and were then placed in bottles containing treatment solutions. The experiment comprised six treatments including T1 silver nitrate (17%), T2 Aspirin (2 mM salicylic acid), T3 rosewater (8%), T4 Sprite (50%), T5 2.5% chitosan, and T6 distilled water as a controlled treatment and each treatment utilized five flowers. The parameters studied were the solution pH, fresh weight of flowers, flower head diameters, flower stem diameter, days for petal discolouration, days for stem discolouration, days for petal drop, days for stem bending, days for leaf wilting, days for petal wilting, days for flower wilting, vase life, relative water uptake, and weight loss. Required measurements were recorded daily.
Statistical Analysis
The experiment was conducted under a completely randomized design (CRD) with five replicates. Recorded data were subjected to analysis of variance (one-way ANOVA) using the software Statistix8.1. Means were compared by LSD at p≤0.05
RESULTS AND DISCUSSION
The primary cause of flower wilting is the depletion of plant nutrients and the plant incapacity to absorb water, resulting in a change in colour and loss of cell turgidity. Flaccid cells cause the flower to have a drooping appearance. To remedy this, an efficient bactericide may be added to the pulse solution. This bactericide will eradicate the buildup of bacteria along the vascular bundle, which obstructs the water passage to the petal.
Ethylene is a gaseous plant hormone involved in various physiological processes, including flower senescence. When a sunflower is cut, it can release ethylene gas as a response to stress and injury. Ethylene promotes petal senescence by accelerating the breakdown of cellular components, such as chlorophyll and proteins, leading to the loss of petal pigmentation and structural integrity. This can contribute to the petal drop in cut sunflowers.
Sunflower petals undergo senescence, a natural ageing and deterioration of plant tissues. During senescence, the cells in the petals break down, leading to wilting and eventual death of the petals. When sunflower stems are cut, air bubbles or embolisms may form within the xylem vessels, which are responsible for transporting water and nutrients throughout the plant. These air bubbles can block water flow, causing discolouration and wilting of the stem.
Cut flowers require nutrients to maintain their vitality. Suppose the vase solution lacks essential nutrients, such as carbohydrates, minerals, or flower food. In that case, the flowers may not sustain themselves properly, resulting in wilting, petal dropping, and overall deterioration. If there are any organic materials, such as leaves or flower debris, in the vase water, they can break down over time. This decomposition process can release compounds that raise the pH of the solution.
The experiment was conducted to improve the vase life of cut sunflowers with six different solutions, including silver nitrate (17%), rosewater (8%), Sprite (50%), chitosan (2.5%), and distilled water as a controlled treatment. The results show that the Sprite solution was the best among other solutions.
Effect of vase solutions on sunflower petal discolouration
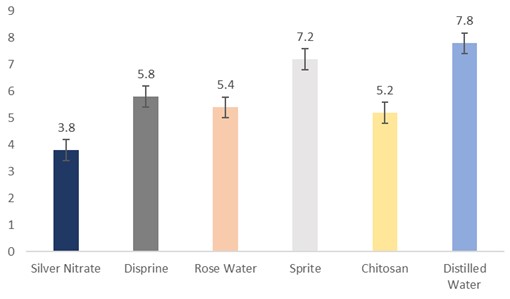
The data regarding days for petal discolouration were subjected to statistical analysis, and the results showed that different holding solutions were significantly different. The cut sunflowers dipped in distilled water took the longest period (7.8 days) days to petal discolour, followed by Sprite (7.2 days), Disprin (5.8 days), rosewater (5.4 days), and chitosan (5.2 days). Meanwhile, the flowers dipped in silver nitrate took the shortest period (3.8 days) to petal discolour (Fig. 1).
Figure 1: Days for petal discoloration of cut sunflower concerning different holding solutions (p≤0.05; S.E. ±0.3916).
Effect of vase solutions on sunflower petal drop
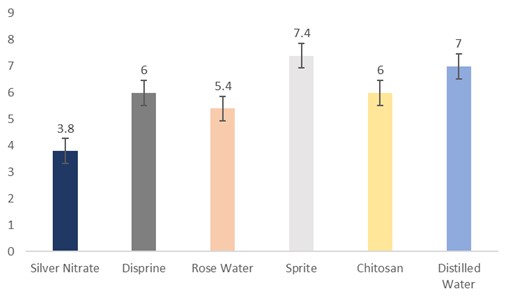
The data regarding days for petal drop was subjected to statistical analysis, and the results showed that the holding solutions were significantly different. The cut sunflowers dipped in Sprite took the longest period (7.4 days) to petal drop, followed by distilled water (7 days), Disprin and chitosan (6 days), and rosewater (5.4 days). Whereas, the flowers dipped in silver nitrate took the shortest period (3.8 days) to petal drop (Fig. 2).
Figure 2: Days for petal drop of cut sunflower concerning different holding solutions (p≤0.05; S.E. ±0.469).
Effect of vase solutions on sunflower stem bending
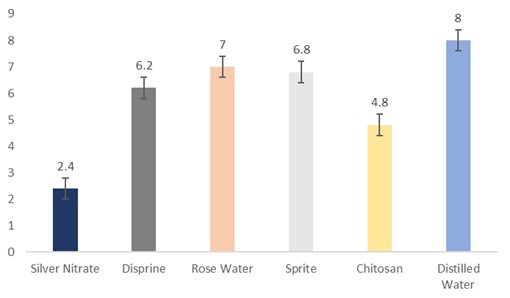
The data regarding days for stem bending were subjected to statistical analysis and the results showed that different holding solutions were significantly different. The cut sunflowers dipped in distilled water took the longest period for stem bending (8 days), followed by rosewater (7 days), Sprite (6.8 days), Disprin (6.2 days), and chitosan (4.8 days). Whereas, the flowers dipped in silver nitrate took the shortest period (2.4 days) for stem bending (Fig. 3).
Figure 3: Days for stem bending of cut sunflower concerning different holding solutions (p≤0.05; S.E. ±0.4).
Effect of vase solutions on sunflower leaf wilting
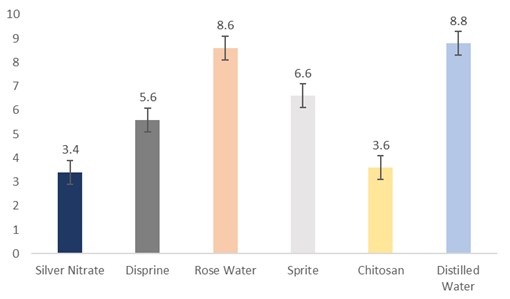
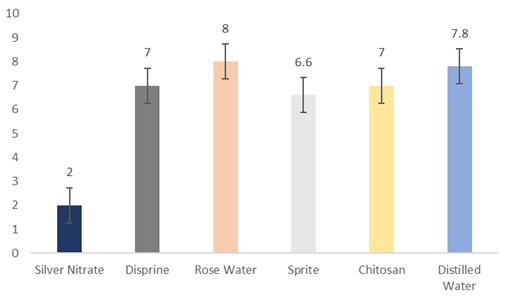
The data regarding days for leaf wilting were subjected to statistical analysis, and the results showed that the holding solutions were significantly different. The cut sunflowers dipped in distilled water (8.8 days) took the highest days, followed by rosewater (8.6 days), Sprite (6.6 days), Disprin (5.6 days), and chitosan (3.6 days). Whereas, the flowers dipped in silver nitrate (3.4 days) took the lowest days for leaf wilting (Fig. 4).
Figure 4: Days for leaf wilting of cut sunflower concerning different holding solutions (p≤0.05; S.E. ±0.4967).
Effect of vase solutions on sunflower stem discoloration
The data regarding days for stem discolouration were subjected to statistical analysis, and the results showed that the holding solutions were significantly different. The cut sunflowers dipped in rosewater took the highest number of days (8 days), followed by distilled water (7.8 days), Disprin and chitosan (7 days), and Sprite (6.6 days). Whereas, the flowers dipped in silver nitrate (2 days) took the lowest days for stem discoloration (Fig. 5).
Figure 5: Days for stem discoloration of cut sunflower concerning different holding solutions (p≤0.05; S.E. ±0.7303).
Effect of vase solutions on sunflower petal wilting
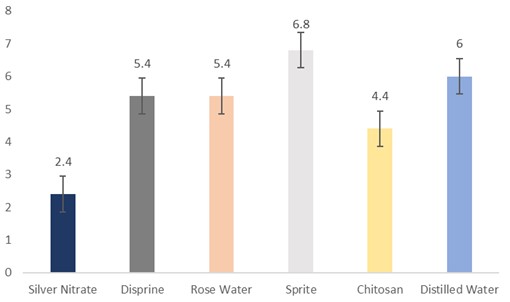
The data regarding days for flower petal wilting were subjected to statistical analysis, and the results showed that different holding solutions were significantly different. The cut sunflowers dipped in Sprite took the highest number of days (6.8 days) for petal wilting, followed by distilled water (6 days), rosewater and Disprin (5.4 days), and chitosan (4.4 days). Whereas, the flowers dipped in silver nitrate (2.4 days) took the lowest days for petal wilting (Fig. 6).
Figure 6: Days for petal wilting of cut sunflower concerning different holding solutions (p≤0.05; S.E. ±0.5416).
Effect of vase solutions on sunflower flower wilting
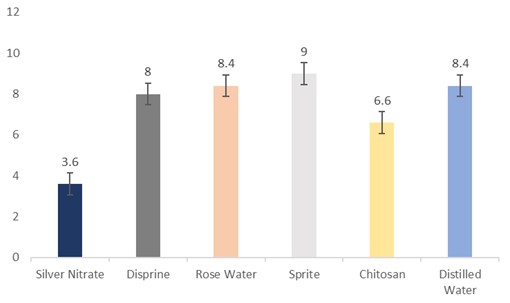
The data regarding days for flower wilting were subjected to statistical analysis, and the results showed that the holding solutions were significantly different. The cut sunflowers dipped in Sprite lasted the longest (9 days), followed by distilled water and rosewater (8.4 days), Disprin (8 days), and chitosan (6.6 days). Conversely, the flowers dipped in silver nitrate (3.6 days) lasted the longest (Fig. 7).
Figure 7: Days for flower wilting of cut sunflower concerning different holding solutions (p≤0.05; S.E. ±0.5292).
Effect of vase solutions on relative water uptake by sunflower
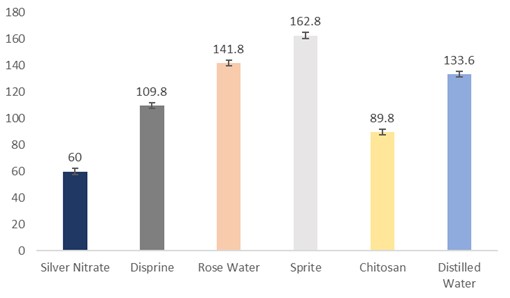
The data regarding the relative water uptake by the flowers were subjected to statistical analysis, and the results showed that the holding solutions were significantly different. The cut sunflowers dipped in Sprite (162.8 mL) had the highest solution uptake, followed by rosewater (141.8 mL), distilled water (133.6 mL), Disprin (109.8 mL), and chitosan (89.8 mL). The flowers dipped in silver nitrate (60 mL) had the lowest solution uptake (Fig. 8).
Figure 8: Relative water uptake by cut sunflower concerning different holding solutions (p≤0.05; S.E. ±2.1307).
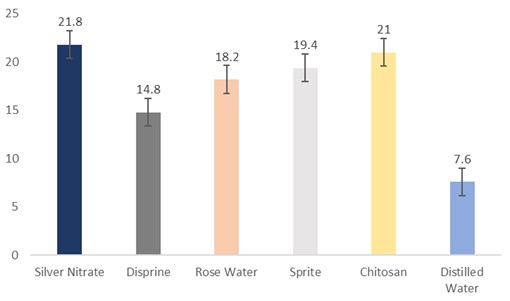
Effect of vase solutions on weight loss of sunflowers
The data regarding flower weight loss were subjected to statistical analysis, and the results showed that different holding solutions had significantly different results. The cut sunflowers dipped in silver nitrate (21.8 g) had the highest weight loss, followed by chitosan (21 g), Sprite (19.4 g), rosewater (18.2 g), and Disprin (14.8 g). Conversely, the flowers dipped in distilled water (7.6) had the lowest weight loss (Fig. 9).
Figure 9: Weight loss of cut sunflower concerning different holding solutions (p≤0.05; S.E. ±1.4306).
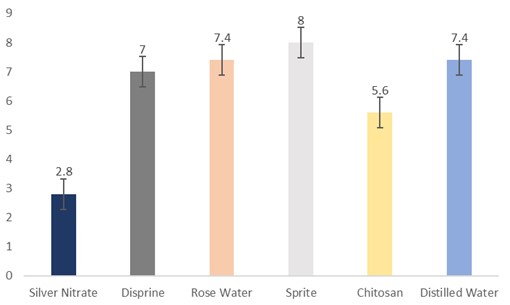
Effect of vase solutions on vase life of sunflowers
The data regarding the vase life of flowers were subjected to statistical analysis and the results showed that different holding solutions were significantly different. The cut sunflowers dipped in Sprite (8 days) had the highest vase life, followed by distilled water and rosewater (7.4 days), Disprin (7 days), and chitosan (5.6 days). Whereas, the flowers dipped in silver nitrate had the lowest vase life (2.8 days) (Fig. 10).
Figure 10: Vase life of cut sunflower concerning different holding solutions (p≤0.05; S.E. ±0.5228).
CONCLUSION
The experiment was conducted to enhance the vase life of cut sunflowers with six different solutions, including silver nitrate (17%), Disprin (2 mM), rosewater (8%), Sprite (50%), chitosan (2.5%), and distilled water as a controlled treatment. The results show that the Sprite solution was the best among other solutions.
Declaration of competing interests
The authors declare no conflict of interest.
Author contribution statement
Rashid Hussain: Conceptualization, Methodology, Software, Validation, Original Draft, Supervision, Project administration. Muhammad Rashid Shaheen: Formal analysis. Muhammad Abdulrehman Iqbal: Writing – Review and Editing. Muhammad Nafees: Supervision. Muhammad Bilal Javed: Writing – Review and Editing.
Acknowledgements
The authors thank the Department of Horticultural Sciences, Faculty of Agriculture and Environment, The Islamia University of Bahawalpur, Punjab, Pakistan, for providing funds to conduct the research reported in this paper.
REFERENCES
Akram, A., Asghar, M.A., Younis, A., Ayyub, C.M., Ahmad, S., Akbar, A.F., Shafiqe, M., Farooq, A., Khan, N.A. and Mushtaq, M.Z. 2021. Effect of plant biostimulants on vase life of Gladiolus grandiflora L. cv. “White Prosperity”. Pakistan Journal of Life and Social Sciences, 19: (2). [Abstract/FREE full text, Google Scholar]
Avilala, D. P., Lakshmi, K. S., Prasad, T. N. V. K. V., Bhaskar, V. V., Ramaiah, M., and Kadiri, L. 2021. Effect of nanosilver integrative plant sciences and silver nitrate on vase life of gerbera (Gerbera jamesonii) cv. Madagascar. The Pharma Innovation Journal. 10 (4): 967-970. [Abstract/FREE full text, Google Scholars]
Azhir, S., Abad, H.H.S. and Mobasser, H.R. 2015. The effect of acetylsalicylic acid and calcium chloride on the vase life of cut flower rose Samurai. Biological Forum, 7(1): 668.
Carrillo-López L.M., Morgado-González, A., Morgado-González, A. 2016. Biosynthesized silver nanoparticles used in preservative solutions for Chrysanthemum cv. Puma. Journal of Nanomaterials. [Abstract/FREE full text, Google Scholar, CrossRef]
Costa, L.C., de Araujo, F.F., Ribeiro, W.S., de Sousa Santos, M.N. and Finger, F.L. 2021. Postharvest physiology of cut flowers. Ornamental Horticulture, 27: 374-385. [Abstract/FREE full text, Google Scholar, CrossRef]
David, L., Harmon, A.C. and Chen, S. 2019. Plant immune responses for guard cells and local responses to systemic defense against bacterial pathogens. Plant Signaling and Behaviour, 14(5): 1588667. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Faust, J.E. and Dole, J.M. 2021. Major Cut Flowers: Cut flowers and foliages. CAB International. Boston, MA, USA, pp. 48-149.
Ferrante, A., Alberici, A., Antonacci, S., Serra, G. 2007. Effect of promoter and inhibitors of phenylalanine ammonia-lyase enzyme on stem bending of cut gerbera flowers. Acta Horticulture, 755: 471-476. [Abstract/FREE full text, Google Scholar, CrossRef]
Ha, S.T., Lim, J.H. and In, B.C., 2019. Extension of the vase life of cut roses by both improving water relations and repressing ethylene responses. Horticultural Science and Technology, 37(1): 65-77. [Abstract/FREE full text, Google Scholar, CrossRef]
Hong-Juan, J., Huan-qing, L. 2015. Chitooligosaccharide prolongs the vase life of cut roses by decreasing reactive oxygen species, Hort. Sci. Technol. 33(3): 383–389. [Abstract/FREE full text, Google Scholar, CrossRef]
Katiyar, D., Hemantaranjan, A. and Singh, B., 2015. Chitosan as a promising natural compound to enhance potential physiological responses in plant: A review. Indian Journal of Plant Physiology, 20: 1-9. [Abstract/FREE full text, Google Scholar]
Ke, M., Ma, Z., Wang, D., Sun, Y., Wen, C., Huang, D., Chen, Z., Yang, L., Tan, S., Li, R. and Friml, J. 2021. Salicylic acid regulates PIN2 auxin transporter hyperclustering and root gravitropic growth via Remorin‐dependent lipid nanodomain organisation in Arabidopsis thaliana. New Phytologist, 229(2): 963-978. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Kılıç, T., Kazaz, S., Şahin, E.G.E. and Uran, M., 2020. Extension of the vase life of cut sunflower by different vase solutions. Ornamental Horticulture, 26(1): 45-50. [Abstract/FREE full text, Google Scholar, CrossRef]
Li, H., Li, H., Liu, J., Luo, Z., Joyce, D. and He, S. 2017. Nano-silver treatments reduced bacterial colonization and biofilm formation at the stem-ends of cut gladiolus ‘Eerde’ spikes. Postharvest Biology and Technology, 123: 102-111. [Abstract/FREE full text, Google Scholar, CrossRef]
Manzoor, A., Ahmad, R. and Naveed, M.S. 2021. Impact of different biocidal compounds for improvement of vase life and quality of cut flowers: A review. Integrative Plant Sciences, 1(1): 1-14. [Abstract/FREE full text, Google Scholar, CrossRef]
Philibert, T., Lee, B.H. and Fabien, N. 2017. Current status and new perspectives on chitin and chitosan as functional biopolymers. Applied biochemistry and biotechnology, 181: 1314-1337. [Abstract/FREE full text, Google Scholar]
Rahman, M.M., Ahmad, S.H., Mohamed, M.T.M. and Rahman, M.Z.A. 2018. Improving the vase life of cut Mokara Red orchid flower using leaf extracts with silver nanoparticles. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 89: 1343-1348. [Abstract/FREE full text, Google Scholar]
Saleem, M., Fariduddin, Q. and Castroverde, C.D.M. 2021. Salicylic acid: A key regulator of redox signalling and plant immunity. Plant Physiology and Biochemistry, 168: 381-397. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Sharif, M.M., Ali, I. and Ahmad, I. 2019. Optimizing planting density for cut Helianthus annuus and Zinnia elegans. Journal of Horticultural Science and Technology, 2: 1-4.
Sun, C., Liu, L., Wang, L., Li, B., Jin, C. and Lin, X. 2021. Melatonin: A master regulator of plant development and stress responses. Journal of Integrative Plant Biology, 63(1): 126-145. [Abstract/FREE full text, Google Scholar, CrossRef]
Uddin, A.F.M.J., Shamsuzzoha, M., Nusrat, A., Taufique, T. and Mehraj, H. 2016. Vase life improvement of yellow gladiolus through different preservative solutions. Journal of Bioscience and Agriculture Research, 10(1): 837-842. [Abstract/FREE full text, Google Scholar]
Wang, C., Dai, S., Zhang, Z.L., Lao, W., Wang, R., Meng, X. and Zhou, X. 2021. Ethylene and salicylic acid synergistically accelerate leaf senescence in Arabidopsis. Journal of Integrative Plant Biology, 63(5): 828-833. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Sunflower, vase life, salicylic acid, chitosan, silver nitrate.
* Corresponding author
Department of Horticultural Sciences, Faculty of Agriculture and Environment, The Islamia University of Bahawalpur, Punjab, Pakistan
Email: abdulbwp325@gmail.com (M.A.R. Iqbal), rashid.hussain@iub.edu.pk (R. Hussain), rashid.shaheen@iub.edu.pk (M.R. Shaheen), muhammad.nafees@iub.edu.pk (M. Nafees), muhammadbilaljaveed372@gmail.com (M.B. Javeed)
This article does not contain any abbreviations to display here.
Received: 02 July 2021
Revised: 26 November 2021
Accepted: 05 December 2021
Published: 31 December 2021
How to Cite
| AMA |
Iqbal MAR, Hussain R, Shaheen MR, Nafees M, Javeed MB. Effect of different vase life-enhancing solutions on vase life of cut sunflower. J Hortic Sci Technol. 2021;4(4):122-127. doi:https://doi.org/10.46653/jhst21044122
|
| MLA |
Iqbal, Muhammad Abdul Rehman, et al. “Effect of Different Vase Life-Enhancing Solutions on Vase Life of Cut Sunflower.” Journal of Horticultural Science & Technology, vol. 4, no. 4, Dec. 2021, pp. 122–27, https://doi.org/10.46653/jhst21044122.
|
| APA |
Iqbal, M. A. R., Hussain, R., Shaheen, M. R., Nafees, M., & Javeed, M. B. (2021). Effect of different vase life-enhancing solutions on vase life of cut sunflower. Journal of Horticultural Science & Technology, 4(4), 122–127. https://doi.org/10.46653/jhst21044122
|
Download Citation (RIGHT CLICK & “SAVE LINK AS”)
This article do not contain any supplementary data.