| Open Access | Peer Reviewed | Review Article |
Callus Culture: A Sustainable Approach for Preserving and Enhancing Cholistan’s Endangered Medicinal Plants for the Herbal Industry

Copyright: © 2023 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution 4.0 International License. J. Hortic. Sci. Technol. © 2023 Pakistan Society for Horticultural Science.
ABSTRACT
Sustainable preservation techniques are necessary due to the rapid depletion of medicinal plants, which are invaluable resources for herbal products. This review focuses on callus culture as a sustainable approach for conserving and enhancing endangered medicinal plants in the Cholistan desert, Pakistan. The Cholistan desert harbours a rich diversity of medicinally important plant species, but agricultural expansion and urbanization threaten their existence. Traditional preservation methods are often tedious and inefficient, emphasizing the need for alternative approaches. Callus culture offers a promising solution for conserving endangered species and enhancing their bioactive compounds. This technique involves the regeneration of plant tissues from explants under controlled conditions. The review discusses the challenges in preserving medicinal plants, the significance of Cholistan’s flora, and the role of callus culture in pharmaceutical industries. It also highlights the importance of research and development in Pakistan to harness the potential of medicinal plants. Furthermore, the review emphasizes the significance of tissue culture techniques in producing bioactive substances and conserving endangered plant species. It discusses the conservation of callus to ensure the sustainable supply of medicinal plant materials to the industry. In summary, the review highlights the significance of callus culture for the sustainable growth of the herbal sector in Cholistan’s endangered medicinal plants.
INTRODUCTION
Medicinal plants are a vital source of herbal products throughout the world, but they are vanishing at a rapid rate (Chen et al., 2016). About 80% of individuals in developing countries rely solely on herbal medicines for primary health care (Chacko et al., 2010). Therefore, their sustainable supply to the herbal and pharmaceutical industry is considered one of the hot issues in the world (Aziz et al., 2018) . This is because most desert plants are in danger of going extinct due to various factors, like climate change, habitat degradation and water scarcity. The Cholistan desert in Pakistan is the world’s seventh largest desert, covering 26,000 km2 (Hameed et al., 2011). A variety of medicinally important chemical compounds can be isolated from the flora of Cholistan desert, including antioxidants, anthocyanidins, flavonoids, phenolics, fatty acids, sterols and steroids, and terpenes and triterpenoids, which will give a breakthrough in terms of improvement in quality of herbal medicines, sustainable marketability and increased profitability (Alamgeer et al., 2018). The increased urbanization and crop cultivation posed a threat to desert flora, resulting in the extinction of various medicinal plant species. The germplasm of endangered plant species could be conserved through seed, field, tissue culture and cryopreservation. Most medicinal plant seeds are recalcitrant, losing viability in long-term storage; field conservation is costly, requires large areas and is damaged by adverse climates. Medicinal plant species which produce recalcitrant seed or do not produce viable seed can be selected for in-vitro storage through the production of callus.
Challenges in the preservation of medicinal plants
It has always been challenging for researchers to preserve the extracted bioactive compounds from plants. Traditional methods of extracting natural compounds from medicinal plants for use in the herbal, pharmaceutical, and chemical industries are tedious, time-consuming, and costly. Moreover, the quantity and quality of extract is also not of good standard. In developed countries, 25% of medicines are made from wild plant species; yet, in developing countries, nearly 80% of people use herbal medicines for primary healthcare (Ekor, 2013). Moreover, reduction in desert plant species and destruction of their habitat has increased the risk of loss of medicinal plants in deserts of China and India (Srujana et al., 2012), likewise in Pakistan. This was primarily due to deforestation and the severity of the climate. Plant extinction rates are currently 100 to 1000 times higher than natural extinction rates, with at least one major medicine being removed from our planet every year (Uhlenbrock et al., 2018). Due to these circumstances, botanicals from the Cholistan deserts were unavailable. World Wildlife Fund (WWF) has listed 15,000 indigenous flora as endangered due to habitat degradation and overgrazing. Likewise, the increasing human population already exhausted 20% of wild resources (Chen et al., 2016).
Research and development in Pakistan
In Pakistan, research and development on medicinal plants is often ignored, even though there are numerous avenues for research in this sector (Asghar et al., 2022). A thriving government policy and regulation, public awareness, and modern biotechnological interventions can result in mass production of these important herbs with long-term metabolite profiles, paving the way for the establishment of a medicinal plant industry in Pakistan and thus supporting the emerging economy (Sher et al., 2014). Storage of medicinal plants as seeds is more difficult because their reproduction biology is not under control as they are wild in nature. Moreover, the bioactive substances are found to be higher in the callus than in field-grown plants (Altemimi et al., 2017). Therefore, clonal propagation is preferred to conserve elite plant species due to the high level of heterozygosity. However, such conserved material is at risk of destruction by natural disasters. It is therefore recommended that endangered medicinal plant materials be conserved through advanced tissue culture approaches (Scherwinski-Pereira et al., 2010), and it is critically required in medicinal plant species (Panis and Lambardi, 2006). Pakistan urgently needs to create a national narrative around medicinal plant preservation and propagation.
The tissue culture techniques have been effectively used in developed countries to regenerate, multiply, and conserve highly valued wild medicinal plants through callus formation (Chokheli et al., 2020). This approach offers a sustainable supply of bioactive compounds regardless of the season, environment, or location (Coelho et al., 2020). Furthermore, it provides a long-term, environment-friendly basis for the industrial production of plant bioactive compounds (Donno et al., 2020).
Tissue culture techniques for medicinal plants
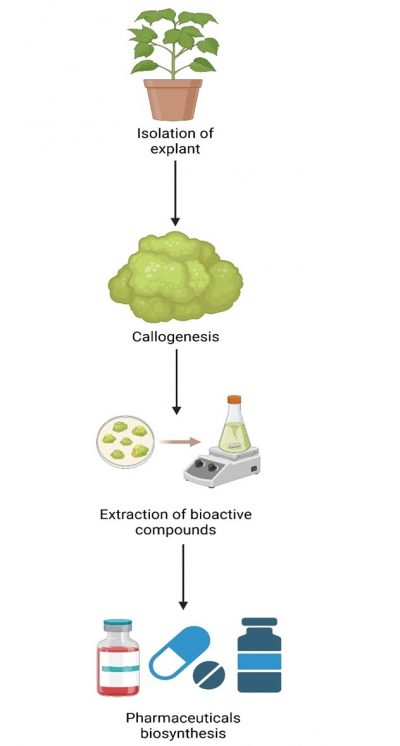
The production of bioactive substances from callus can further be increased through advancements in cell line selection, cell permeabilization, and biotransformation (Marisol et al., 2016). Bahawalpur’s arid region encompasses a wide area of hot Cholistan desert with great seasonal changes, contributing to the extinction of the region high-value medicinal plant species (Malik et al., 2015). Therefore, understanding in-vitro conservation techniques is imperative to preserve the enriched medicinal flora of Cholistan and increase the sustainable production of antioxidants, phenolic and other natural products through callus production to boost the pharmaceutical and herbal industry of Pakistan (Fig. 1). Moreover, the outcome of the project will promote research and development activities in academics, researchers and other stakeholders of the industry. The present study intends to identify the endangered species of Cholistan medicinal plants and then compare the production of bioactive substances in vitro and in vivo. For that purpose, the optimization of culture media for callus production, as well as its multiplication and storage, were reviewed (Fig. 2). Masses of callus were used to extract bioactive compounds. These optimized techniques of callus induction and multiplication will boost the pharmaceutical industry.
Figure 1: Schematic representation of the process of callus culture for preserving and enhancing endangered medicinal plants. The process begins with the isolation of explants from target plants, followed by callogenesis (formation of callus tissue under controlled conditions). Bioactive compounds are then extracted from the callus tissue for further pharmaceutical biosynthesis, contributing to the production of high-value herbal medicines and products.
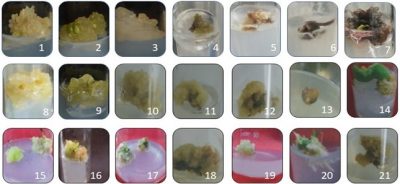
Figure 2. Medicinal plants species with successful calli induction after optimization of MS media 1, Carnation (Dianthus caryophyllus); 2, Goji berry (Lycium barbarum); 3, Harmal (Penganum harmala); 4, Bitter cucumber (Cucumis callosus); 5, Datura (Datura stramonium); 6, Stevia (Stevia rebaudiana); 7, Jujube (Ziziphus jujube); 8, Vaccaria (Vaccaria hispanica); 9, Indian rennet (Withania coagulans); 10, Desert Pink (Sesuvium sesuvioides); 11, Kapok bush (Aerva javanica); 12, False Amaranth (Digera muricata); 13, Ak (Calotropis procera); 14, Bitter apple (Citrullus colocynthis); 15, Fagonia (Fagonia bruguieri); 16, Seetzenia (Seetzenia lanata); 17, Gokshur (Tribulus terrestris); 18, Kair (Capparis decidua); 19, Sand button (Neurada procumbens); 20, Bindweed (Convolvulus prostrartus); 21, Camelthorn (Alhagi maurorum).
REVIEW OF LITERATURE
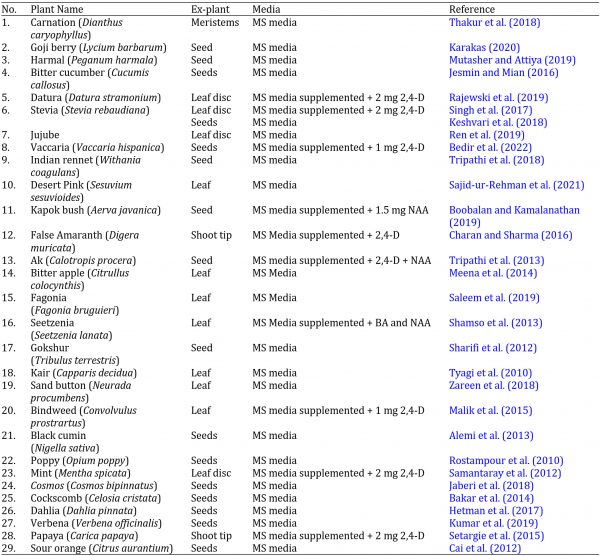
A large number of individuals have been using herbal remedies as primary healthcare in developing countries (Myers et al., 2000). However, rapid urbanization, habitat destruction, ruthless herb collection, pollution, and other anthropogenic activities diminished the ecosystem’s medicinal plants (Shaik et al., 2011), and most wild plant species in desert zones of the world, including Cholistan are on the verge of extinction To prevent the future extinction of endangered medicinal plants, the conservation of plant genetic resources has long been recognized as an essential component of biodiversity conservation (Khan et al., 2021). As a result, it is proposed that callus be conserved not only from various medicinal plant species but also utilized for quantitative and qualitative enhancement of antioxidant and phenolic extraction on a long-term basis. The advancement in plant tissue culture provides enormous potential for the preservation and multiplication of rare medicinal plants (Robert et al., 2012). In-vitro propagation is the process of propagating chosen genotypes in a laboratory setting (Govarthanan et al., 2015). Several types of explants, like roots or leaves pieces, can be subjected to a growing medium with essential nutrients to produce calli. Recent studies have reported the in vitro growth of various medicinal plants (Table 1). As a result, attempts have been made to improve the in-vitro culture of valuable medicinal plants that are highly threatened.
Table 1: List of plants species cultured in-vitro with protocol: tap water washing à distilled water washing à Tween-20 + 5% NaClO for 2 min à 70% ethanol treatment for 2 min à distilled water washing à double distilled water washing.
Role of cell culture in pharmaceutical industry
Plants have been used for therapeutic purposes worldwide since prehistoric times (Chandran et al., 2020). Most tropical and subtropical medicinal plant species have recalcitrant seeds, which reduces their viability in storage and makes them unsuitable for traditional preservation (Dhaniya and Parihar, 2019). Furthermore, in situ conservation (field gene banks) is a simple technique, it is always vulnerable to natural disasters, insects/pests, and diseases (Zair, 2020). So, it is critical to have access to a coordinated system (tissue culture techniques) to preserve plant germplasm that can serve as the foundation for global agriculture (Scherwinski-Pereira et al., 2010). Rapid multiplication and production of medicinal plants under disease-free conditions can be accomplished using cell culture, nodal culture, and other tissue culture techniques (Cruz-Cruz et al., 2013). In vitro propagation of medicinal plant species offers the safe exchange of plant material, the establishment of a large collection of calli through a multiplication procedure that takes up little space, and the facilitation of molecular and ecological investigations in various parts of the world (Tandon and Kumaria, 2005).
Plant organs like shoots, leaves, and roots are produced through calluses and the differentiation of meristems by adjusting the concentrations of plant growth hormones in a nutrient medium through organogenesis (Oseni et al., 2018). Worldwide, a lot of research success has been achieved in vitro propagation and conservation of various endangered medicinal plant species like Mandevilla velutina, used as an anti-inflammatory agent and snake bites. Explant (nodal segments) of this crop was successfully inoculated on MS media by supplementing different concentrations of zeatin, benzyl adenine, and thidiazuron (Biondo et al., 2007).
There is a great commercial importance of plant secondary metabolites containing anthocyanins, alkaloids and flavonoids group of organic compounds. The extraction of these compounds is limited due to various ecological and climatic conditions worldwide (Praveen and Murthy, 2011). Plant tissue culture technology can extract secondary metabolites more efficiently than conventional approaches (Praveen et al., 2010). Moreover, the production of these metabolites depends on the age of plants and is tissue-specific (Nasim et al. 2010). Commercial propagation of Capparis decidua in the field is difficult because of less ability to produce viable seeds; moreover, their seed germination is very poor in the soil, so there is a need to develop micropropagation approaches for this species (Sottile et al., 2021). In vitro regeneration of Alhagi maurorum (rare halophytic medicinal plant) to produce phenotypic clones to extract bioactive compounds was successfully done in tissue culture practices (Agarwal et al., 2015). In 3 to 4 weeks of culture of Dicoma tomentosa and Alhagi maurorum, calli production started from leaves in tested medium MS-5 after optimization of Auxins and cytokinin concentrations (Dhaniya and Parihar, 2019).
Conservation of cell culture products
Traditionally, the biodiversity of medicinal plants was conserved through in situ and ex-situ practices; however, there is always a threat to habitat devastation and change in the natural environment. Therefore, it is recommended that in situ conservation of endangered medicinal plant species is insufficient and that other advanced means of conservation be followed (Mir et al., 2021). Advancements in plant biotechnology and molecular studies provide effective tools to support and improve the conservation of plant diversity, done in tissue culture practices (Cruz-Cruz et al., 2013). Conservation of callus or cell culture is necessary not only for sustainable supply of medicinal plant material to industry for drug making but also to save these species from extinction. Various conservation methods and temperatures are followed depending on conservation objectives and type of plant species. In vitro, slow growth through extending subculture practices from several months to years, depending on plant species, is termed medium-term conservation. Conservation of callus of medicinal plant species in liquid nitrogen (-196°C) is the only safe and cost-effective technique for long-term conservation of diverse plant species. Moreover, these cryopreservation techniques have also been successfully applied to shoot tips to eliminate systemic plant pathogens (Cruz-Cruz et al., 2013). Tissue culture protocols are successfully used to preserve callus or organs as clones instead of seeds, saving the genetic background of endangered medicinal plant species due to various biotic or abiotic stresses (Shahzad et al., 2017). Biondo et al. (2007) established long-term storage of Brazilian endangered medicinal plant species (Mandevilla velutina) through in vitro culture of micro-cuttings and transferring it to different culture media and concluded that MS media with reduced nutrient concentration (MS/2) improved slow regeneration of explants.
Significance of Cholistan’s medicinal plant species
There are lots of medicinal plants naturally growing in the desert zone of Bahawalpur (Naz et al., 2010). The most prevalent medicinal plants of the Cholistan region include Peganum harmala L., which belongs to the family (Nitrariaceae) known as Syrian rue, Wild rue (common names) and Harmal (local name). It can tolerate extensive drought by losing leaves and branches during the moisture-free months (Gouja et al., 2014). It is a perennial desert shrub that shows different activities in biological sciences, including antimicrobial activity (Alkhalifah, 2013), antioxidant and cytotoxic activities (Badria et al., 2007), anti-ulcer, anti-inflammatory activities and hypoglycemic activities (El-Hawary and Kholief, 1990). Several plant species, including Aerva javanica, Capparis decidua, Cleome brachycarpa, Dipterygium glaucum, Gisekia pharnacioides and Suaeda fruticosa are used for vermicidal and anthelmintic properties against intestinal worms or antimicrobial activities against bacteria and other microorganisms (Alkhalifah, 2013). The flora of the Cholistan desert contains several chemical compounds that have been isolated and recognized to have medicinal significance, including terpenes, triterpenoids, phenolics, and antioxidants. Allopathic medicines are often expensive and out of reach for many locals (Sharif et al., 2011).
Bathu (Chenopodium album) has been declared an endangered herb, native to Europe and Western Asia, as narrated in the book “Food in China”. It has been a food source for different old civilizations as it was likely grown in Neolithic Europe (7000-1700 BC). It usually grows straight up to a height of around 30 cm. Its stem often has red, purple, or green stripes. The leaves in cultivated varieties vary greatly from simple, deltoid, and rhomboid to lanceolate form and length, ranging from 10-15 cm with an entire top surface and a toothed or uneven lower surface. The petioles are frequently as long as the thick blade, measuring 1 to 1.3 cm. The opposite leaves can be very different in shape. The early leaves are serrated, roughly diamond-shaped, 3-7 cm long and 3-6 cm broad. They are located close to the base of the plant. It has been spotted in a dark green colour with a smooth undersurface. The leaves are wax-coated, un-wettable and mealy in shape. Flowers are radial and symmetrical and grow in small cymes on a deeply branched inflorescence, 10-40 cm long, consisting of shining black seeds. pollen contributed to high fever-like allergies (Wiart, 2006; Pande and Pathak, 2010). It shows a source of anthelmintic agents and used medicinally in different countries for a source of many potent and strong drugs. Ethanol leaf extract of plant has been found to reveal antibacterial activity on all Gram (+) and Gram (-) bacteria (Korcan et al., 2013). It is a fast growing “weedy annual plant” with about 150 species occurring almost in different parts of the world (Colombo and Bosisio, 1996). It is used as a food crop in Northern India and is also given to the animals as their daily base feed. The leaves contain about 0.76% fats, 3.9% proteins, 8.93% carbohydrates and 3% ash, calcium, phosphorus and vitamin A (Parekh and Chanda, 2007). Iron and fibre are also usually present in very small quantities to do any harm (Rojas et al., 2003).
Haloxylon salicornicum, locally known as “Khar” or “Lana”, a member of Chenopodiaceae, is a widespread shrub in different desert regions of Pakistan (Raza et al., 2014). It is a perennial, upright, leafless shrub with many branches. The branches and stem are jointed and pale yellow in colour. The joints split into two small triangle points, replacing leaves, flowers, and fruits. It is a fodder plant with high salt content and is mostly grazed by the camels, which is better for reclaiming the soil. Its extract is used to wash clothes. Some residents also contend that this plant is toxic due to inaccurate knowledge, and most people use it as an external remedy for local population insect stings (Ashraf et al., 2012). Its decoction is known to have anti-inflammatory and antibacterial properties in folk medicine. Traditional healers use it to treat intestinal ulcers (Fatima et al., 2019; Shafi et al., 2002). It is utilized in multiple forms as fodder, food, fuel, and medicinal plants in different countries (Singh et al., 2022). The stems and leaves of this plant are used to make animal feed, which contains various minerals and chemical components (Ashraf et al., 2012). In Oman, its stem is used for dyeing wool in traditional weaving (Abdullah et al., 2020). It is also a densely branched pale diffuse shrub. Its stem is almost leafless and glabrous, and it secretes thick fluid on the cut wounds. The inflorescence is pale greenish, like a small cup with spikes. Wings like fruits are brownish (Arora et al., 2010). It is also used as fodder (Khan and Qaiser, 2006). Other reported uses in various countries are burned plants used in washing clothes, in glass, in the soap industry, and in dyeing clothes and medicines (Baber et al., 2018). Mollugo cerviana is a flowering plant species, locally known as thread stem carpet weed. It grows as a weed in many dry and sandy habitats on different continents. It is an annual herb producing a thin, erect stem up to about 20 centimetres tall. The narrow, waxy leaves are up to 1.5 centimetres long, linear in shape, and arranged in whorls around the stem. The inflorescence is a loose umbel of tiny flowers, each made of whitish, petal-like sepals <2 mm long and no true petals. The annual, glabrous herb grows up to 12 cm tall, branchless glaucous, in whorls of 8 from the rootstock. It has antimicrobial and anti-inflammatory properties (Valarmathi et al., 2012). The extracts of this plant can act as a uterine stimulant and antiseptic, and it is also used to treat jaundice (Valarmathi et al., 2011). Besides this, in India, it is identified as a suppressor of stomach ache and to promote vaginal discharge in childbirth. It also clears the eyesight and reduces body odour (Aglin, 2022). Decoction of flowers and tender shoots is used to treat diaphoretic impact. C-glycolsyl flavonoids compounds are also present in this species (Dewangan et al., 2022). Its crude extract contains alkaloids, saponins, flavonoids, glycosides tannins, triterpenoids, and phenolic groups, while the ethyl acetate fraction contains active constituents like saponins, glycoside, triterpenoids and steroids (Valarmathi, 2012). This plant species belongs to the family Capparidaceae. This plant is typically found in arid environments, such as wastelands and foothills. It is highly branched, typically grows between 6-10 meters, thorny and climbing shrub with 2.4 meters of stem diameter. Delicate branches with waxy blooms; rough, corky and grey bark, covered with 3-7 mm long pointed, straight orange-yellow stipular thorns. The leaves on the young branches are linear, 1-2 cm long, caduceus with a short rigid apex that resembles a pickle, with extremely short petiole (Kirtikar and Basu, 1999). The flowers in lateral corymbs are crimson, pink, or occasionally yellow, ovoid or globose, 1-2 cm in diameter, dull red berries with a hard, woody, 1-2 mm thick brownish rind; gynophores, 1.5-2 cm long, originating from the enlarged base of the thalamus; pedicel slender and fragile; tip with a little point resembling the style scar; bitter flavour; powerful, foul-smelling scent; globose seeds, 2-5 mm in diameter (Sulakshana and Raju, 2019). This plant is used to treat lumbago, hiccough, emmenagogue cough, and asthma and as a tonic, carminative, appetizer, alexipharmic, and aphrodisiac. The young leaves and upper branches are used as powder as an antidote to poison and for boils, eruptions, and swellings. They are very effective in relieving toothache when chewed (Kirtikar and Basu, 1999). The fruits are useful in cardiac scrapes. The fruits and tender flower buds are soused. Fruits are consumed either ripe or green. Useful in treating mudpack paralysis, enlarged spleen issues, and intestinal worm infestation (Srivastava et al., 2023).
CONCLUSION
Callus culture offers a promising and sustainable approach to preserving and enhancing endangered medicinal plant species in the Cholistan region. It ensures their survival, enhances their therapeutic value, and secures their role in the herbal industry. Moreover, callus culture provides a platform for further research on metabolic engineering and secondary metabolite production, opening new avenues for pharmaceutical and nutraceutical applications.
Declaration of competing interests
The authors declare no conflict of interest.
Author contribution statement
Muhammad Wasim Haider, Muhammad Nafees: Critically reviewed the literature and prepared the manuscript draft. Ishtiaq Ahmad, Asad Masood, Muhammad Samsam Raza, Izhar Ul Haq, Muhammad Ahmad Saeed, Aqsa Shabbir, Umar Farooq: Performed editing and reviewing of the manuscript.
REFERENCES
Abdullah, Khan, S.M., Pieroni, A., Haq, Z.U and Ahmad, Z. 2020. Mazri (Nannorrhops ritchiana (Griff) Aitch.): A remarkable source of manufacturing traditional handicrafts, goods and utensils in Pakistan. Journal of Ethnobiology and Ethnomedicine, 16(45): 1-13. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Agarwal, T., Gupta A.K., Patel, A.K. and Shekhawat N.S. 2015. Micropropagation and validation of genetic homogeneity of Alhagi maurorum using SCOT, ISSR and RAPD markers. Plant Cell Tissue and Organ Culture, 120: 313-323. [Abstract/FREE full text, Google Scholar, CrossRef]
Aglin, A.A. 2022. Medicinal effects of Mollugo cerviana – A brief review. Current Aspects in Pharmaceutical Research and Development, 7: 45-51. [Abstract/FREE full text, Google Scholar, CrossRef]
Alamgeer, A.M.U., Ahsan, H., Hasan, U.H. and Chaudhary M.A. 2018. Traditional medicines of plant origin used for the treatment of inflammatory disorders in Pakistan: A review. Journal of Traditional Chinese Medicine, 38(4): 636-656. [Abstract/FREE full text, PubMed, Google Scholar]
Alemi, M., Sabouni F., Sanjarian F., Haghbeen K. and Ansari S. 2013. Anti-inflammatory effect of seeds and callus of Nigella sativa L. extracts on mix glial cells with regard to their thymoquinone content. AAPS PharmSciTech, 14: 160-167. [Abstract/FREE full text, Google Scholar]
Alkhalifah, D.H. 2013. In-vitro antibacterial activity of ethanol extract of Calligonum comosum plant against four human pathogens in Saudi Arabia. International Journal of Plant Animal and Environmental Sciences 3: 170-175. [Abstract/FREE full text, Google Scholar]
Altemimi, A., Lakhssassi, N., Baharlouei, A., Watson D.G. and Lightfoot D.A. 2017. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants, 6(4): 42. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Arora, J., Goyal, S. and Ramawat, K.G. 2010. Biodiversity, biology and conservation of medicinal plants of Thar Desert. In: Ramawat, K.G. (eds.). Desert plants: Springer, Berlin/Heidelberg, pp. 3-36. [Abstract/FREE full text, Google Scholar, CrossRef]
Asghar, S., Hasan, S., Khan, M.N., Hussain, T., Khalid, S., Siddique, I.M. and Akhtar, J. 2022. Performance evaluation of selected cherry cultivars under climatic conditions of Murree hills. Plant Cell Biotechnology and Molecular Biology, 23: 37-43. [Abstract/FREE full text, Google Scholar, CrossRef]
Ashraf, M.A., Karamat, M., Shahnaz, K., Wajid, A. and Yusoff, I. 2012. Study of chemical and mineral constituents of Haloxylon salicornicum collected from Cholistan Desert, Bahawalpur, Pakistan. Wulfenia Journal, 19: 306-327. [Abstract/FREE full text, Google Scholar]
Aziz, M.A., Adnan, M., Khan, A.H., Shahat, A.A., Al-Said, M.S. and Ullah, R. 2018. Traditional uses of medicinal plants practiced by the indigenous communities at Mohmand Agency, FATA, Pakistan. Journal of Ethnobiology and Ethnomedicine, 14: 2. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Baber, A., Ahamd, S., Rehman, T., Ul Sabaha N. and Arshad, M.A. 2018. A review on phytochemical analysis and ethnobotanical uses of Haloxylon stocksii. RADS Journal of Pharmacy and Pharmaceutical Sciences, 6: 162-167. [Abstract/FREE full text, Google Scholar]
Badria, F.A., Ameen, M. and Akl, M.R. 2007. Evaluation of cytotoxic compounds from Calligonum comosum L. growing in Egypt. Zeitschrift für Naturforschung C, 62(9-10): 656-660. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Bakar, D.A., Ahmed, B.A. and Taha, R.M. 2014. In vitro callus induction and plant regeneration of Celosia argentea-an important medicinal plant. Brazilian Archives of Biology and Technology, 57(6): 860-866. [Abstract/FREE full text, Google Scholar, CrossRef]
Bedir, H., Ari, E., Vural, G.E. and Seguí-Simarro, J.M. 2022. Effect of the genotype, explant source, and culture medium in somatic embryogenesis and organogenesis in Vaccaria hispanica Rauschert. Plant Cell Tissue and Organ Culture, 150: 329-343. [Abstract/FREE full text, Google Scholar, CrossRef]
Biondo, R., Souza, A.V., Bertoni, B.W., Soares, A.M., Franca, S.C. and Pereira, A.M.S. 2007. Micropropagation, seed propagation, and germplasm bank of Mandevilla velutina Woodson. Scientia Agricola, 64: 263-268. [Abstract/FREE full text, Google Scholar, CrossRef]
Boobalan, S. and Kamalanathan, D. 2019. Spermidine influences enhanced micropropagation and antibacterial activity in Aerva javanica (Burm. F.) Shult. Industrial Crops and Products, 137:187-196. [Abstract/FREE full text, Google Scholar, CrossRef]
Cai, Z., Kastell, A., Knorr, D. and Smetanska, I. 2012. Exudation: An expanding technique for continuous production and release of secondary metabolites from plant cell suspension and hairy root cultures. Plant Cell Reports, 31: 461-477. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Chacko, S.M., Thambi, P.T., Kuttan, R. and Nishigaki, I. 2010. Beneficial effects of green tea: a literature review. Chinese Medicine, 5: 13. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Chandran, H., Meena, M., Barupal, T. and Sharma, K. 2020. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnology Reports, 26: 1-10. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Charan, P.D. and Sharma, K.C. 2016. Floral diversity of Thar desert of western Rajasthan, India. Journal of Phytological Research, 29: 55-71. [Abstract/FREE full text, Google Scholar]
Chen, S.L., Yu, H., Luo, H.M., Wu, Q., Li, C.F and Steinmetz, A. 2016. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chinese Medicine, 11: 37. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Chokheli, V.A., Dmitriev, P.A., Rajput, V.D., Bakulin, S.D., Azarov, A.S., Varduni, T.V., Stepanenko, V.V., Tarigholizadeh, S., Singh, R.K., Verma, K.K. and Minkina, T.M. 2020. Recent development in micropropagation techniques for rare plant species. Plants, 9(12): 1733. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Coelho, N., Gonçalves, S. and Romano, A. 2020. Endemic Plant Species Conservation: Biotechnological approaches. Plants, 9(3): 345. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Colombo, M.L. and Bosisio, E. 1996. Pharmacological activities of Chelidonium majus L. (Papaveraceae). Pharmacological Research, 33(2): 127-134. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Cruz-Cruz, C.A., Gonzalez-Arnao, M.T. and Engelmann F. 2013. Biotechnology and conservation of plant biodiversity. Resources, 2: 73-95. [Abstract/FREE full text, Google Scholar]
Dewangan, N.R., Agrawal, A. and Ahirwar, B. 2022. Novel therapeutic approach of Mollugo cerviana (L.) ser plant: A review. Research Journal of Pharmacy and Technology, 15: 3280-3284. [Abstract/FREE full text, Google Scholar, CrossRef]
Dhaniya, S. and Parihar, S.K. 2019. In vitro callus induction and multiplication of inter-nodal explants in plants Dicoma tomentosa and Alhagi maurorum. Journal of Drug Delivery and Therapeutics, 9: 212-219. [Abstract/FREE full text, Google Scholar, CrossRef]
Donno, D., Turrini, F., Boggia, R., Guido, M., Gamba, G., Mellano, M.G., Riondato, I. and Beccaro, G.L. 2020. Sustainable extraction and use of natural bioactive compounds from the waste management process of Castanea spp. Bud-Derivatives: The FINNOVER Project. Sustainability, 12(24): 10640. [Abstract/FREE full text, Google Scholar, CrossRef]
Ekor, M. 2013. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Frontiers in Pharmacology, 4: 177. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
El-Hawary, Z. and Kholief, T. 1990. Biochemical studies on some hypoglycemic agents (II) effect of Calligonum comosum extract. Archives of Pharmacal Research, 13: 113-116. [Abstract/FREE full text, Google Scholar, CrossRef]
Fatima, I., Munawar, M., Iqbal, S. and Sadaf, Z. 2019. Ethno-medicinal uses of wild herbs and shrubs of Tehsil Yazman, Punjab, Pakistan. Pakistan Journal of Agricultural Sciences, 56(3): 735-741. [Abstract/FREE full text, Google Scholar, CrossRef]
Gouja, H., Fernandez, A.G., Garnatje, T., Raies, A. and Neffati, M. 2014. Genome size and phylogenetic relationships between the Tunisian species of the genus Calligonum (Polygonaceae). Turkish Journal of Botany, 38(1): 13-21. [Abstract/FREE full text, Google Scholar, CrossRef]
Govarthanan, M., Rajinikanth, R., Kamala-Kannan, S. and Selvankumar, T. 2015. A comparative study on bioactive constituents between wild and in-vitro propagated Centella asiatica. Journal of Genetic Engineering and Biotechnology, 13: 25-29. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Hameed, M., Ashraf, M., Al-Quriany, F., Nawaz, T., Sajid, M., Ahmad, A., Younis, A. and Naz, N. 2011. Medicinal Flora of the Cholistan desert: A review. Pakistan Journal of Botany, 43: 39-50. [Abstract/FREE full text, Google Scholar]
Hetman, J., Lukawska-Sudol, S., Pudelska K. and Parzymies, M. 2017. The effect of the cutting method on rooting of Dahlia pinnata Cav. cuttings. Acta Scientiarum Polonorum Hortorum Cultus, 16: 110. [Abstract/FREE full text, Google Scholar]
Jaberi, M., Azadi, P., Gharehyazi, B., Khosrowchahli, M., Sharafi, A., Aboofazeli, N. and Bagheri, H. 2018. Silver nitrate and adenine sulphate induced high regeneration frequency in the recalcitrant plant Cosmos bipinnatus using cotyledon explants. The Journal of Horticultural Science and Biotechnology, 93: 204-208. [Abstract/FREE full text, Google Scholar, CrossRef]
Jesmin, R., and Mian, M.A.K. 2016. Callus induction and efficient plant regeneration in Cucumber (Cucumis sativus L.). Journal of Bioscience and Agriculture Research, 9: 796-803. [Abstract/FREE full text, Google Scholar, CrossRef]
Karakas, F.P. 2020. Efficient plant regeneration and callus induction from nodal and hypocotyl explants of goji berry (Lycium barbarum L.) and comparison of phenolic profiles in calli formed under different combinations of plant growth regulators. Plant Physiology and Biochemistry, 146: 384-391. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Keshvari, T., Najaphy, A., Kahrizi, D. and Zebarjadi, A. 2018. Callus induction and somatic embryogenesis in Stevia rebaudiana Bertoni as a medicinal plant. Cellular and Molecular Biology, 64: 46-49. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Khan, A., Shah, A.H. and Ali, N. 2021. In vitro propagation and phytochemical profiling of a highly medicinal and endemic plant species of the Himalayan region (Saussurea costus). Scientific Reports, 11: 1-13. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Khan, M.A. and Qaiser, M. 2006. Halophytes of Pakistan: Characteristics, distribution and potential economic usages. In: Khan, M.A.G. Kust, S., Barth, H.J and Boer, B. (eds.). Springer, Netherland, pp. 129-153. [Abstract/FREE full text, Google Scholar]
Kirtikar, K.R. and Basu, B.D. 1999. Indian Medicinal Plants, International Book Distributers, Dehradun (2nd Ed.) pp. 195-199. [Google Scholar]
Korcan, S.E., Aksoy, O., Erdogmus, S.F., Cigerci, I.H. and Konuk, M. 2013. Evaluation of antibacterial, antioxidant and DNA protective capacity of C. album ethanolic leaf extract. Chemosphere, 90: 374-379. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Kumar, A., Anam, A., Singh, A.K. and Naseem, M. 2019. In vitro regeneration of plantlets from nodal culture of Verbena officinalis L. Mendel International Journal, 36: 13-20. [Google Scholar]
Malik, S., Ahmad, S., Sadiq, A., Alam, K., Wariss, H.M., Ahmad, I., Hayat, M.Q., Anjum, S. and Mukhtar, M. 2015. A comparative ethno-botanical study of Cholistan (an arid area) and Pothwar (a semi-arid area) of Pakistan for traditional medicines. Journal of Ethnobiology and Ethnomedicine, 11: 31. [Abstract/FREE full text, Google Scholar, CrossRef]
Marisol, O.V., Howat, S., Hong, S., Jang, M.O., Jin, Y.W., Lee, E.K. and Loake, G.J. 2016. Plant cell culture strategies for the production of natural products. BMB Reports, 49: 149-158. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Meena, M.C., Meena, R.K., Meena, R. and Meena, V.P. 2014. Biochemical changes of various metabolites in Citrullus colocynthis (Linn) Schrad. International Journal of Current Pharmaceutical Research, 6: 45-48. [Abstract/FREE full text, Google Scholar]
Mir, T.A., Jan, M., Khare, R.K. and Bhat, M.H. 2021. Medicinal plant resources: Threat to its biodiversity and conservation strategies. In: Aftab, T. and Hakeem, K.R. (eds.). Medicinal and Aromatic Plants. Springer, Cham. [Abstract/FREE full text, Google Scholar]
Mutasher, H.H. and Attiya, H.J. 2019. Induced callus from seedlings of Peganum harmala L. and studying harmine compound concentration in vitro and in vivo by GC analysis. Iraqi Journal of Science, 60(7): 1442-1451. [Abstract/FREE full text, Google Scholar, CrossRef]
Myers, N., Mittermeier, R.A., Mittermeier, C.G., Fonseca, G.A.B. and Kent, J. 2000. Biodiversity hotspots for conservation priorities. Nature, 403: 853-858. [PubMed, Google Scholar, CrossRef]
Nasim, S.A., Dhir, B., Kapoor, R., Fatima, S., Mahmooduzzafar and Mujib, A. 2010. Alliin production in various tissues and organs of Allium sativum grown under normal and sulphur-supplemented in vitro conditions. Plant Cell Tissue and Organ Culture, 101: 59-63. [Abstract/FREE full text, Google Scholar, CrossRef]
Naz, N., Hameed, M., Ahmad, M.S.A., Ashraf, M. and Arshad, M. 2010. Is soil salinity one of the major determinants of community structure under arid environments? Community Ecology, 11: 84-90. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Oseni, O.M., Pande, V. and Nailwal, T.K. 2018. A review on plant tissue culture, A technique for propagation and conservation of endangered plant species. International Journal of Current Microbiology and Applied Sciences, 7: 3778-3786. [Abstract/FREE full text, Google Scholar, CrossRef]
Pande, M. and Pathak, A. 2010. Preliminary pharmacognostic evaluations and phytochemical studies on leaf of Chenopodium album (Bathua Sag). Asian Journal of Experimental Biological Sciences, 1: 91-95. [Abstract/FREE full text, Google Scholar]
Panis, B. and Lambardi, M. 2006. Status of cryopreservation technologies in plants (crops and forest trees). pp 61-78. In: Ruane, J. and Sonnino, A. (eds.), The Role of Biotechnology in Exploring and Protecting Agricultural Genetic Resources. Food and Agriculture Organization of the United Nations, Rome. [Abstract/FREE full text, Google Scholar]
Parekh, J. and Chanda, S.V. 2007. In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turkish Journal of Biology, 31: 53-58. [Abstract/FREE full text, PubMed, Google Scholar]
Praveen, N. and Murthy, H.N. 2011. Effects of macroelements and nitrogen source on biomass accumulation and withanolide – A production from cell suspension cultures of Withania somnifera (L.) Dunal. Plant Cell Tissue and Organ Culture, 104: 119-124. [Abstract/FREE full text, Google Scholar, CrossRef]
Praveen, N., Naik, P.M., Manohar, S.H., Nayeem, A. and Murthy, H.N. 2010. In vitro regeneration of Brahmi shoots using semisolid and liquid cultures and quantitative analysis of bacoside A. Acta Physiologiae Plantarum, 31: 723-728. [Abstract/FREE full text, Google Scholar, CrossRef]
Rajewski, A.C., Elkins, K.B., Henry, A., Van Eck, J. and Litt, A. 2019. In vitro plant regeneration and Agrobacterium tumefaciens-mediated transformation of Datura stramonium (Solanaceae). Applications in Plant Sciences, 7: 01220. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Raza, M.A., Younas, M., Buerkert, A. and Schlecht, E. 2014. Ethno-botanical remedies used by pastoralists for the treatment of livestock diseases in Cholistan desert, Pakistan. Journal of Ethnopharmacology, 151: 333-342. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Ren, H., Du, X., Li, D., Zhao, A. and Wang, Y. et al. 2019. An efficient method for immature embryo rescue and plant regeneration from the Calli of Ziziphus jujuba Lengbaiyu. The Journal of Horticultural Science and Biotechnology, 94: 63-69. [Abstract/FREE full text, Google Scholar, CrossRef]
Robert, J., Ravi, B.X. and Louis, C. 2012. An efficient in-vitro plant regeneration of Dipteracanthus prostratus (Poir.) Nees. – a medicinal herb. Asian Pacific Journal of Tropical Biomedicine, 2(2): S484-S487. [Abstract/FREE full text, Google Scholar, CrossRef]
Rojas, R., Bustamante, B., Bauer, J., Fernandez, I., Alban, J. and Lock, O. 2003. Antimicrobial activity of selected Peruvian medicinal plants. Journal of Ethnopharmacology, 88: 199-204. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Rostampour, S., Sohi, H.H. and Dehestani, A. 2010. In vitro regeneration of Persian poppy (Papaver bracteatum). Biologia, 65: 647-652. [Abstract/FREE full text, Google Scholar, CrossRef]
Sajid-ur-Rehman, M., Ishtiaq, S., Khalil-ur-Rehman, M. and Bauer, R. 2021. Pharmacognostic screening, physico-chemical and cytotoxic potential of Sesuvium sesuvioides (Fenzyl) Verdc. Pakistan Journal of Pharmaceutical Sciences, 34: 20. [Abstract/FREE full text, PubMed, Google Scholar ]
Saleem, R., Shinwari, Z.K., Ali, A., Malik, A., Shahzad, M.S., Butt, M. and Ali, Q. 2019. Comparative in vitro anti-oxidant and anti-fungal potential profiles from methanol extract of Fagonia indica, Fagonia bruguieri and Fagonia paulayana. International Journal of Botany Studies, 4: 69-76. [Abstract/FREE full text, Google Scholar]
Samantaray, A., Sial, P. and Kar, M. 2012. Micro-propagation and biochemical analysis of Spear Mint (Mentha spicata). Indian Journal of Innovations and Developments, 489-493. [Abstract/FREE full text, Google Scholar]
Scherwinski-Pereira, J.E., Costa, F.H.S., Camillo, J., Silva, D.B., Alves, R.B.N. and Vieira, R.F. 2010. Tissue culture storage of Brazilian medicinal plants germplasm. In: Baricevic et al. (eds.), Proc. 4th IS on Breeding Research on Medicinal and Aromatic Plants. Acta Horticultural. 860:15-20. [Abstract/FREE full text, Google Scholar]
Setargie, A., Mekbib, F. and Abraha, E. 2015. In vitro propagation of papaya (Carica papaya L.). World Journal of Agricultural Sciences, 11: 84-88. [Abstract/FREE full text, Google Scholar]
Shafi, P.M., Rosamma, M.K. and Jamil, K. 2002. Antibacterial activity of Syzygium cumini and Syzygium travancoricum leaf essential oils. Fitoterapia, 73: 414-416. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Shahzad, A., Parveen, S. Sharma, S. Shaheen, A. Saeed, T. Yadav, V. Akhtar, R. Ahmad Z. and Upadhyay, A. 2017. Plant tissue culture: Applications in plant improvement and conservation. In Plant Biotechnology: Principles and Applications; M. Abdin, U. Kiran, A. Ali (eds.) Springer: Singapore, pp. 37-72. [Abstract/FREE full text, PubMed, Google Scholar]
Shaik, S., Singh, N. and Nicholas, A. 2011. Cytokinin-induced organogenesis in Lessertia (Sutherlandia frutescens L.) using hypocotyl and cotyledon explants affects yields of L-canavanine in shoots. Plant Cell Tissue and Organ Culture, 105: 439-446. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Shamso, E., Rabei, S. and Hamdy, R. 2013. Identification keys and numerical studies of Zygophyllaceae and allied families in Egypt. Assiut University Journal of Botany, 42: 79-106. [Abstract/FREE full text, Google Scholar]
Sharif, A., Ahmed, E., Malik, A., Mukhtar-ul-Hassan, Munawar, M.A., Farrukh, A., Nagra, S.A., Anwar, J., Ashraf, M. and Mahmood, Z. 2011. Antimicrobial constituents from Aerva javanica. Journal of the Chemical Society of Pakistan, 33: 439-443. [Abstract/FREE full text, Google Scholar]
Sharifi, S., Sattari, T.N., Zebarjadi, A., Majd, A. and Ghasempour, H.R. 2012. Enhanced callus induction and high-efficiency plant regeneration in Tribulus terrestris L., an important medicinal plant. Journal of Medicinal Plants Research, 6: 4401-4408. [Abstract/FREE full text, Google Scholar, CrossRef]
Sher, H., Aldosari, A., Ali, A. and de Boer, H.J. 2014. Economic benefits of high value medicinal plants to Pakistani communities: An analysis of current practice and potential. Journal of Ethnobiology and Ethnomedicine, 10: 71. [Abstract/FREE full text, PubMed, Google Scholar, CrossRef]
Singh, D.N., Bohra, J.S., Tyagi, V., Singh, T., Banjara, T.R. and Gupta, G. 2022. A review of India fodder production status and opportunities. Grass and Forage Science, 77: 1-10. [Abstract/FREE full text, Google Scholar, CrossRef]
Singh, M., Saharan, V., Dayma, J., Rajpurohit, D., Sen, Y. and Sharma, A. 2017. In vitro propagation of Stevia rebaudiana (Bertoni): An overview. International Journal of Current Microbiology and Applied Sciences, 6: 1010-1022. [Abstract/FREE full text, Google Scholar, CrossRef]
Sottile, F., Caltagirone, C., Peano, C., Del Signore, M.B. and Barone, E. 2021. Can the Caper (Capparis spinosa L.) still be considered a difficult-to-propagate crop? Horticulturae, 7(9): 316. [Abstract/FREE full text, Google Scholar, CrossRef]
Srivastava, R.P., Kumar, A., Gupta, K. and Saxena, G. 2023. Role of aromatic and medicinal plants in phytoremediation of heavy metal pollutants. In Phytoremediation Potential of Medicinal and Aromatic Plants, pp. 100-115. CRC Press, Boca Raton, Florida, USA. [Abstract/FREE full text, Google Scholar, CrossRef]
Srujana, S.T., Babu, K.R. and Rao, B.S.S. 2012. Phytochemical investigation and biological activity of leaves extract of plant Boswellia serrata. Pharmaceutical Innovation, 1: 22-46. [Abstract/FREE full text, PubMed, Google Scholar]
Sulakshana, M. and Raju, A.J.S. 2019. Pollination ecology of three ecologically valuable carpetweed herbs, Mollugo cerviana, M. nudicaulis and M. pentaphylla (Molluginaceae). Journal of Threatened Taxa, 11: 13334-13349. [Abstract/FREE full text, Google Scholar, CrossRef]
Tandon, P. and Kumaria, S. 2005. Prospects of plant conservation biotechnology in India with special reference to northeastern region. In Biodiversity: Status and Prospects. Tandon, P. and Kumaria, S. (eds.). Norasa Publishing House: New Delhi, India, pp. 79-91. [Abstract/FREE full text, Google Scholar]
Thakur, K. and Kanwar, K. 2018. In vitro plant regeneration by organogenesis from leaf callus of carnation, Dianthus caryophyllus L. cv. Master. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 88:1147-1155. [Abstract/FREE full text, Google Scholar]
Tripathi, D., Rai, K.K., Rai, S.K. and Rai, S.P. 2018. An improved thin cell layer culture system for efficient clonal propagation and in vitro withanolide production in a medicinal plant Withania coagulans Dunal. Industrial Crops and Products, 119: 172-182. [Abstract/FREE full text, Google Scholar, CrossRef]
fusion_menu_anchor name=”tripathi_2013″ class=””][/fusion_menu_anchor]
Tripathi, P.K., Awasthi, S., Kanojiya, S., Tripathi, V. and Mishra, D.K. 2013. Callus culture and in vitro biosynthesis of cardiac glycosides from Calotropis gigantea (L.) Ait. In Vitro Cellular and Developmental Biology-Plant, 49: 455-460. [Abstract/FREE full text, Google Scholar, CrossRef]
Tyagi, P., Khanduja, S. and Kothari, S.L. 2010. In vitro culture of Capparis decidua and assessment of clonal fidelity of the regenerated plants. Biologia Plantarum, 54: 126-130. [Abstract/FREE full text, Google Scholar, CrossRef]
Uhlenbrock, L., Sixt, M., Tegtmeier, M., Schulz, H., Hagels, H., Ditz, R. and Strube, J. 2018. Natural products extraction of the future—Sustainable manufacturing solutions for societal needs. Processes, 6: 177. [Abstract/FREE full text, Google Scholar, CrossRef]
Valarmathi, R. A. Rajendran, S. Akilandeswari, V.N. Indu latha and M.V.L. Nagaswetha, M.V.L. 2011. Hepatoprotective efficacy of Mollugo cerviana Linn. against carbon tetrachloride induced liver damage in rats. International Journal of Pharmaceutical Sciences and Research, 2: 176-179. [Abstract/FREE full text, Google Scholar]
Valarmathi, R., Rajendran, A. and Akilandeswari, S. 2012. Preliminary phytochemical screening and antimicrobial activity studies on Mollugo cerviana. International Journal of Pharmaceutical and Chemical Sciences, 1: 404-406. [Abstract/FREE full text, Google Scholar]
Wiart, C. 2006. Medicinal plants of the East Pacific. Drugs for the Future? World Scientific Publishing Co Pte Ltd. [Abstract/FREE full text, Google Scholar]
Zair, W. 2020. Conservation of crop wild relatives’ diversity in the Fertile Crescent (PhD dissertation, University of Birmingham). [Abstract/FREE full text, Google Scholar]
Zareen, S., Zahra, S.S., Mehmood, A., Asadullah, M. and Muhammad, A. 2018. In vitro propagation of Neurada procumbensl L. (Chipri Booti): An endangered medicinal plant from Cholistan desert. Pakistan Journal of Agricultural Research, 31(1): 1-6. [Abstract/FREE full text, Google Scholar, CrossRef]
Bioactive compounds, callus culture, Cholistan, conservation, endangered plant species, herbal industry, medicinal plants.
* Corresponding author
a Plant Tissue Culture Laboratory, Department of Horticultural Sciences, Faculty of Agriculture and Environment, The Islamia University of Bahawalpur, Bahawalpur 63100, Pakistan
b Technical Support-Crop Protection Development, Syngenta, Multan 60000, Pakistan
c Department of Horticulture, Faculty of Agriculture, Ordu University, Ordu 52200, Türkiye
Email: muhammad.nafees@iub.edu.pk (M. Nafees)
This article does not contain any abbreviations to display here.
Received: 26 July 2023
Revised: 14 November 2023
Accepted: 25 November 2023
Published: 31 December 2023
How to Cite
| AMA |
Haider MW, Nafees M, Ahmad I, et al. Callus culture: A sustainable approach for preserving and enhancing Cholistan’s endangered medicinal plants for the herbal industry. J Hortic Sci Technol. 2023;6(4):41-49. doi:https://doi.org/10.46653/jhst23064041
|
| MLA |
Haider, Muhammad Wasim, et al. “Callus Culture: A Sustainable Approach for Preserving and Enhancing Cholistan’s Endangered Medicinal Plants for the Herbal Industry.” Journal of Horticultural Science & Technology, vol. 6, no. 4, Dec. 2023, pp. 41–49, https://doi.org/10.46653/jhst23064041.
|
| APA |
Haider, M. W., Nafees, M., Ahmad, I., Masood, A., Raza, M. S., Haq, I. U., Saeed, M. A., Shabbir, A., & Umar Farooq. (2023). Callus culture: A sustainable approach for preserving and enhancing Cholistan’s endangered medicinal plants for the herbal industry. Journal of Horticultural Science & Technology, 6(4), 41–49. https://doi.org/10.46653/jhst23064041
|
Download Citation (RIGHT CLICK & “SAVE LINK AS”)
This article do not contain any supplementary data.